Conversion disorder is a neuropsychiatric condition at the interface of neurology and psychiatry. Clinically trained neurologists see approximately 30% of outpatients for medically unexplained illness,
1 and up to 18% of patients with unexplained illness are diagnosed with functional neurologic symptoms.
2 Despite frequent clinical exposure to conversion disorder, after identifying functional signs, including distractibility and inconsistency, without objective deficits, neurologists are uncomfortable in the psychiatrist’s chair.
3 The lack of a conceptual framework through which to understand this disorder is exemplified by a neurologist’s stating “Well, I don’t really know….I suppose it may be their way of dealing with problems they can’t solve”
3. It is the collective clinical experience of the authors that part of the challenge for physicians in working with these patients lies in the absence of an accepted neurobiological framework through which to understand the clinical phenotype of functional neurological symptoms.
Advanced functional neuroimaging techniques now allow access to neural-system dysfunction in conversion disorder. Despite multiple neuroimaging studies and reviews on the topic,
4 a brain-based conceptual model through which to understand functional neurological disturbances has yet to be recognized. In this article, conversion disorder is first historically contextualized to allow for the integration of neurologic and psychiatric concepts with an emerging neurobiology. Second, the neurobiology of the disorder is explored by reviewing functional neuroimaging findings in the most well-studied subset of patients (functional unilateral motor and somatosensory disturbances). Thereafter, conversion disorder is positioned among the “unawareness” disorders in neuropsychiatry, and
functional unawareness is suggested as a useful neurobiological framework through which to understand this illness.
Historical Perspective: Origins of Hysteria
FND was first known in the medical literature as “hysteria,” and it has engaged prominent individuals in the history of medicine, neurology, and psychiatry. Although initially described in ancient Greece with gynecologic (“wandering womb”) themes and later as demonic possession after the rise of Christian civilization,
6 hysteria was medicalized in the 19th century by the French neurologist Jean-Martin Charcot, through his study of patients at the Salpêtrière Hospital. Charcot recognized hysteria as an acceptable, neurologically diagnosable condition, stating “the neurological tree has its branches; neurasthenia, hysteria, epilepsy, all the types of mental conditions, progressive paralysis, gait ataxia.”
7Reclassification of hysteria as a psychiatric disorder began with the writings of Sigmund Freud, an Austrian neurologist and founder of psychoanalysis. Freud argued, “the aetiology was to be sought in sexual factors.”
8 He coined the term “conversion hysteria” and described a process whereby “the affective idea is converted into a physical phenomenon.”
8 Whereas Freud described a transformation of psychic conflict into somatic symptoms, Pierre Janet, a French psychologist, suggested that “hysteria is a form of mental depression characterized by retraction of the field of personal consciousness and a tendency to the
dissociation and emancipation of the system of ideas and functions that constitute personality.”
9 Thus, by the late 19th/early 20th century, FND was an accepted neurologic and psychiatric condition.
The FND clinical syndrome is now recognized to occur more often in women, with symptom onset often presenting during the teens or early-20s. Patients have an elevated rate of Axis I comorbidity, and symptoms are generally acute in onset, of short duration with multiple reoccurrences, and commonly occur after a psychological stress. As originally described by Freud, patients may exhibit an unusually calm demeanor regarding their symptoms (“la belle indifférence”), whereas others exhibit a high degree of emotionality. Childhood sexual and physical trauma may be reported. In functional weakness, movements, if performed, are slow, tentative, and nonsustained. Functional somatosensory deficits may manifest with nonphysiological demarcations, such as immediately left of the sternum, or may exhibit fluctuating boundaries.
Neural formulations of FND have emerged in the last few decades. Whitlock, in 1967, described hysteria as a neuropsychiatric disorder involving attentional dysregulation, characterized by “selective depression of awareness of a bodily function.”
10 Sierra and Berrios, in 1999, proposed altered attention and awareness, mediated in part by inferior parietal cortex dysfunction, as a neurobiological model for FND.
11 Lateralized right-hemispheric dysfunction with relative preservation of a narrative, interpretative left hemisphere, has also been suggested as a neural explanation for FND, and implies an overlap with delusional disorders.
12,13 Also, theories applied to the broader category of somatoform disorders have emphasized affect dysregulation and somatic amplification as important mediators of disease.
14,15Neuroimaging of Unilateral Motor/Somatosensory FND
Over the past 10–15 years, the neurobiology of unilateral motor and somatosensory functional neurologic disturbances has advanced with the use of functional neuroimaging techniques, including
99Tc-SPECT, FDG-PET, and fMRI. Prefrontal inhibition of primary motor/somatosensory cortex,
16–19 intentional disturbances,
20,21 attentional dysregulation,
22–24 impaired action authorship recognition,
25 and affective disturbances
26,27 are major neural processes implicated in the neuroscience of FND.
Inhibition
The first published neuroimaging study in FND was performed on a 32-year-old woman with panic attacks, depression, and recent marital discord, who developed left-sided weakness (preserved finger movements, with arm weakness and foot clumsiness) and paresthesias.
18 99Tc-SPECT blood flow patterns in response to left median nerve stimulation revealed right frontal cortex hyperperfusion and right parietal cortex (including primary somatosensory cortex) hypoperfusion, only during symptomatic periods. In related studies, medial prefrontal cortex (PFC) hyperactivation was replicated and further localized to the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and ventromedial PFC.
16,17,19 Medial PFC inhibition of unimodal primary somatosensory and motor cortices was postulated to explain functional numbness and weakness.
17,18Intention
Impairment in the generation of motor intention was also hypothesized for patients with FND.
20 Three men with functional arm weakness (two, left-sided) and previous depression exhibited left dorsolateral PFC hypometabolism compared with healthy subjects on FDG-PET during performance of joy stick movements. Functional weakness was suggested to reflect a disturbance in motor intention planning. This notion has been supported by evidence of altered functional connectivity between the dorsolateral PFC and sensorimotor areas in patients with FND,
21 and further delineation of a role for the dorsolateral PFC in motor intention generation and cognitive control of motor behavior.
28,29Attention
A third neurocognitive function, attention, was explored, using
99Tc-SPECT in seven patients with functional unilateral hemiparesis/hypoesthesia (4, left-sided; 5, with depressed mood) during rest and passive bilateral vibratory stimulation.
24 Contralateral thalamic and basal-ganglia blood-flow reductions were observed only in symptomatic patients during sensory stimulation. Impairments in striato-thalamic components of attentional and motivational neural networks were proposed as mediators of FND. Also, in related investigations,
30 patients with unilateral motor FND exhibited bilateral striatal/pallidal and right-thalamic volume reductions. Thalamic dysfunction was replicated in functional anesthesia,
23 and a lack of striato-thalamic circuit activation was also found in patients with functional weakness.
19In a unique case, a 56-year-old, right-handed woman with emotional distress related to marital discord presented with transient functional left arm and leg weakness, hypoesthesia
and functional left-hemispatial neglect.
31 fMRI obtained while she was symptomatic, during performance of a line-bisection task, showed right ACC hyperactivity relative to healthy subjects. Thus the perigenual ACC (pACC), posterior parietal cortex (PPC),
18 striatum, and thalamus, regions implicated in attentional neurobiology, exhibited dysfunctional activity in FND patients.
Action Authorship
Disturbances of motor intention awareness and self-agency have also been suggested for patients with FND. Voon and colleagues
25 examined fMRI blood-oxygen level-dependent (BOLD) patterns in eight FND patients (two with major depression, three with generalized anxiety disorder) with positional, predominantly unilateral, unexplained tremors as compared with volitional movements. The right temporoparietal junction (TPJ) was less active during unexplained movements, and reduced functional connectivity occurred between the right TPJ and bilateral sensory/motor cortex, ACC, medial PFC, and right superior parietal lobule. Given the roles of the TPJ and adjacent regions (PPC/superior temporal gyrus) in motor intention awareness and self-agency perception,
32–35 diminished right TPJ activity and aberrant connectivity were suggested to explain FND patients’ inability to recognize themselves as the authors of their actions.
Affective Disturbances
Two imaging studies directly probed affective (limbic) circuit dysfunction in FND patients. A scripted, traumatic memory provocation fMRI study was administered to a woman with selective amnesia for a romantic break-up and functional right hemiparesis/hypoesthesia. fMRI showed right amygdala, ACC, parietal cortex, and inferior frontal activations during cued recall of repressed memories, as compared with readily-recalled events.
26 Amygdalar activation was proposed as a biomarker of heightened emotional salience for repressed memories. A second fMRI study examined affective facial processing in 16 mixed-phenotype, hyperkinetic, FND patients with anxiety and depression, compared with healthy subjects. Patients with hyperkinetic FND showed increased amygdalar activation to happy faces and increased amygdalar functional connectivity with the supplementary motor area (SMA).
27 Motor disinhibition resulting from heightened limbic–SMA interactions via striato-pallidal-thalamic projections were proposed as mechanisms of limbic influences on motor actions.
25 These studies supported the idea of amygdalar and ACC dysfunction in patients with FND.
Having reviewed prefrontal-mediated inhibition, intentional disturbances, inattention, impaired action-authorship recognition, and affective dysregulation as major neural processes implicated in the neurobiology of functional neurologic disturbances, the following section frames unilateral functional motor and somatosensory disturbances as “disorders of unawareness.”
Unawareness and Right-Hemisphere Dysfunction
The neglect syndrome is defined as a right-brain/left-body unawareness disorder characterized by impaired ability to report, respond, or orient to novel or salient stimuli; it is associated with right-hemispheric lesions.
36,37 Subtypes include sensory (somatosensory, visual, auditory), motor, hemispatial, and personal neglect.
38 Of particular interest is motor neglect, characterized by impaired motor intention generation and exemplified by limb underuse, hypokinesia, and inability to sustain motor movements (motor impersistence), despite the absence of corticospinal system damage.
38,39 Notably, functional weakness resembles motor neglect. Structural studies in motor-neglect stroke patients identify a pattern of right hemisphere-predominant frontal, parietal, striatal and thalamic lesions,
40–44 and implicate cortico-cortical (frontal-parietal) and cortico-subcortical pathways in lesional motor neglect.
Concepts related to motor neglect include motor intention awareness (the conscious recognition of the desire to move) and self-agency (the sense that we are the authors of our own movements).
34 Early behavioral studies examining predictions of motor action
45 and hand position
46 suggested that individuals used internal sensory predictions to anticipate motor actions, a process termed forward modeling or corollary discharge.
32,45–47 Forward modeling allows the central nervous system to maintain accurate performance based on the predicted sensory consequences before the actual processing of sensory afferent signals. Furthermore, observations have shown that the predictability of sensory consequences following motor actions correlates with perceived self-agency.
48 Forward modeling, awareness of motor intention, and self-agency may be related concepts that involve common or interrelated neural circuits. Also, aberrant forward modeling has been extended to other modalities and proposed as an explanation for neuropsychiatric disturbances, including auditory hallucinations and delusions of control in schizophrenic patients experiencing their own internal dialogue or actions as “other.”
47A role for the PPC in forward modeling has been suggested by lesional and functional neuroimaging studies. Lesions of the superior parietal lobule have been linked to time-dependent decrements in sustained arm motor function and proprioceptive unawareness,
49 and updates of limb posture recruited bilateral superior parietal cortex activations in an fMRI spatial-pointing task.
50 These results supported a role for the PPC, particularly the superior parietal lobule, in real-time dynamic internal sensorimotor integration, a prerequisite for predictive forward-modeling.
Studies also implicated the PPC in motor intention awareness and self-agency. Patients with right inferior parietal lobule (IPL) lesions exhibited a shortened latency between conscious recognition of impending action and motor execution. This suggested a deficit in motor intention awareness before the imminent release of motor action.
34,35 Interestingly, patients undergoing intraoperative IPL cortical stimulation experienced the conscious desire for motor action at low intensity and an experience of motor accomplishment at high intensity.
33 This implied that perception of action authorship involved similar neural networks to that of motor intention awareness. In further support of this concept, patients with IPL lesions falsely attributed examiner-made, complex hand movements as their own,
51 and bilateral angular gyrus activations occurred in individuals making agency determinations.
52 Thus, the PPC, a node in the large-scale brain network disturbed in lesional neglect (including motor neglect), participates in forward-modeling, motor-intention awareness, and self-agency perceptions.
34,53,54Somatosensory neglect (e.g. tactile extinction to double simultaneous stimulation in the absence of lateralized somatosensory deficits) is difficult to isolate clinically, and frequently coexists with primary somatosensory disturbances, motor weakness, and hemispatial neglect, limiting its study in isolation. However, extrapolation from hemispatial neglect, the most studied neglect syndrome subtype, allows an inference into the neurobiology of somatosensory unawareness. Primarily on the basis of lesional analyses, a distributed, right hemisphere dominant, cortical and subcortical network for hemispatial neglect has been suggested to include the PPC, TPJ, superior temporal gyrus, ACC, frontal eye fields, inferior frontal gyrus, striatum, and thalamus.
55–63 Somatosensory and motor neglect, therefore, may share similar neural substrates.
FND: A Functional Unawareness Syndrome
Functional neural circuit disturbances in motor and somatosensory FND overlap with the cortico-cortical and cortico-subcortical pathways implicated in lesional motor and somatosensory neglect. A number of cross paradigm studies in patients with functional limb weakness demonstrated patterns of neural dysfunction in right-greater-than-left PPC (extended to include the TPJ),
18,25 ACC,
17,26,64 striatum, and thalamus.
19 Functional neuroimaging studies in patients with functional somatosensory deficits exhibited similar dysfunction in attentional regions, again including right-greater-than-left parietal cortex,
18 ACC,
17,23,26,31 striatum,
24 and thalamus.
23,24 In further support of an overlapping neural substrate between FND and lesional motor and somatosensory unawareness, a case of transient functional left-of-midline hemibody anesthesia was described in a patient with a right parietal infarct.
65 Psychogenic non-epileptic seizures may occur more often in patients with right-hemispheric lesions or right-hemispheric dysfunction on electroencephalogram.
66 Lastly, left-sided bodily deficits have traditionally been considered more frequent in FND,
67 although a recent metaanalysis failed to show this effect.
68Using a best-fit approach to synthesize neuroimaging and phenomenological data,
functional somatosensory unawareness and
functional motor unawareness are suggested as major contributors in the pathophysiology of unilateral somatosensory and motor related FND (
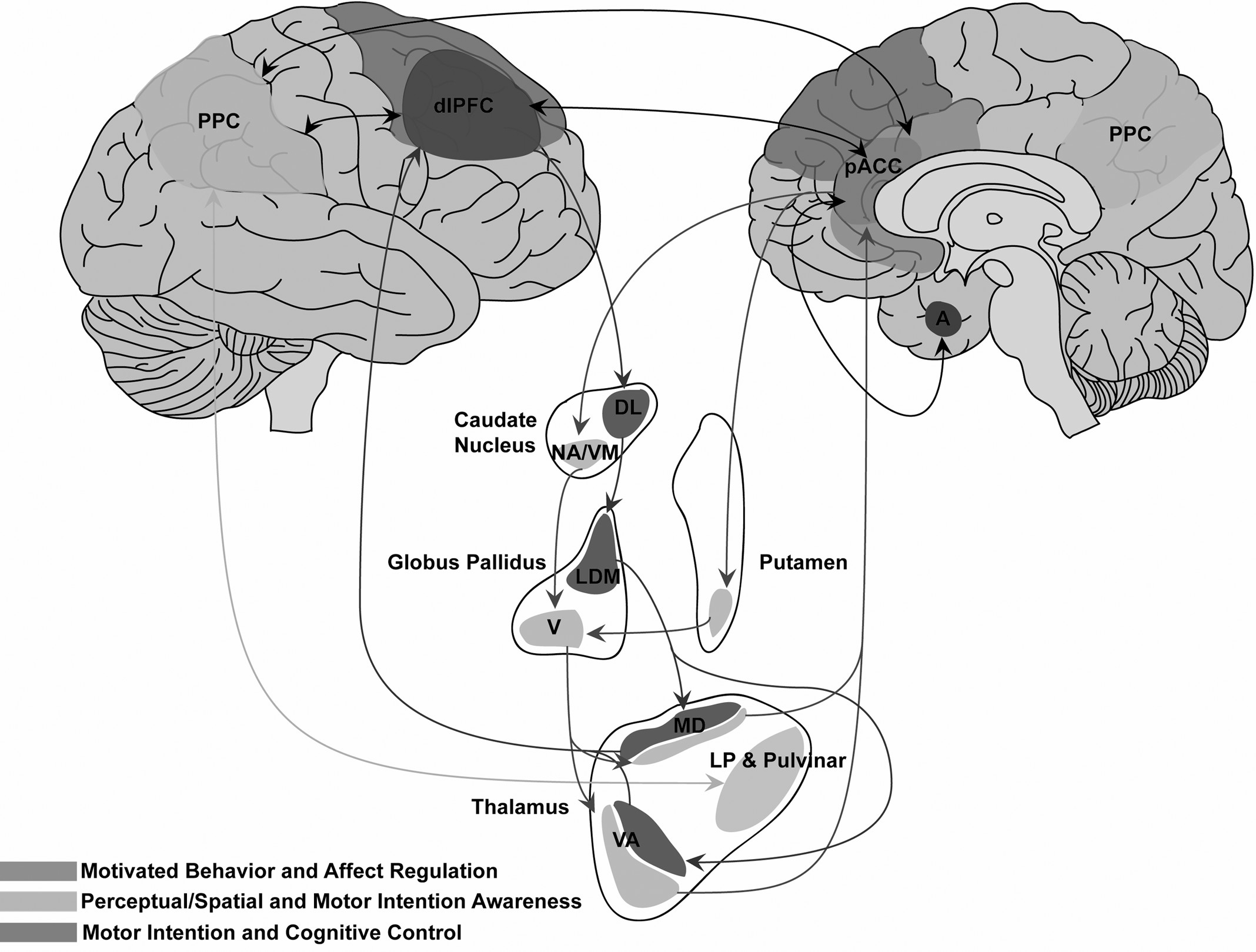
Figure 1). Clinical and neural features of functional disturbances share a common denominator with lesional neglect: unawareness. Individual variation across unilateral motor and somatosensory FND phenotypes may be accounted for, in part, by relative contributions of prefrontal, posterior parietal, and subcortical components. Furthermore, pACC-subcortical and PPC-subcortical pathways may provide complementary, but distinct, unawareness contributions.
69,70 Disturbances of motivated behavior/motor control/affect regulation may arise preferentially from pACC–subcortical pathways (including pACC–amygdalar circuits), whereas attentional and perceptual miscalculations may arise from PPC–subcortical dysfunction.
69,71–73 Reciprocal cortico–cortical connections among the pACC, PPC, and dorsolateral PFC facilitate interactions among circuits mediating affect regulation, awareness, intention, and cognitive control.
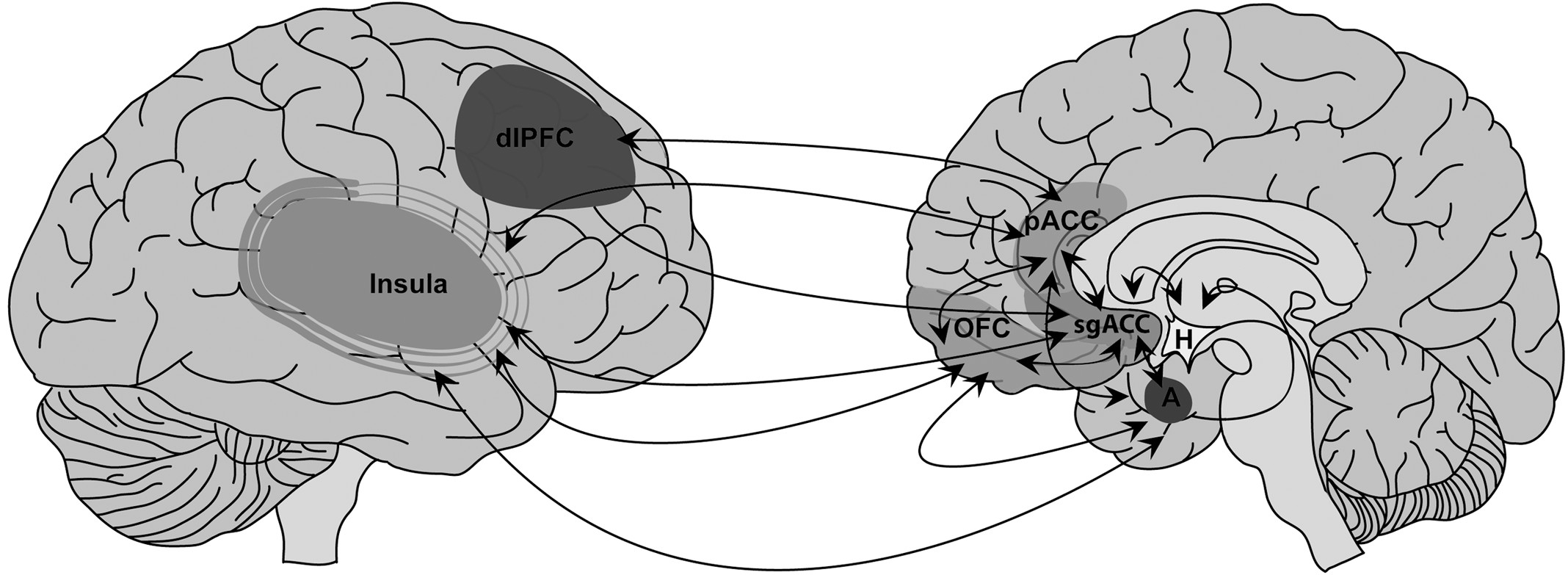
74,75In this conceptualization, in addition to the PPC, an important role is postulated for the pACC in functional motor/somatosensory neglect, given its dual cognitive and emotional functions mediated by structural connections with the posterior parietal, premotor, dorsolateral prefrontal, OFC, subgenual ACC (sgACC), and medial-temporal cortices.
76–80 Human and animal models also implicate the ACC in stress-related, maladaptive experience-dependent neuroplastic change. For example, patients with posttraumatic stress disorder (PTSD) after childhood sexual trauma exhibited functional and morphologic abnormalities in the ACC, as compared with healthy subjects.
81,82 Chronic stress in animal models induced dendritic spine reduction in the ACC and hippocampus, with parallel enhanced dendritic arborization in the amygdala.
83,84 Reciprocal top-down (ACC) and bottom-up (amygdala) sites of aberrant experience-dependent neuroplastic change, and medial/lateral and dorsal/ventral PFC interactions specific to FND patients require more exploration in the context of extended emotional/affective regulation–neural circuit explorations (
Figure 2). Furthermore, the OFC, implicated in social-emotional evaluation and behavioral control in the context of changing contingencies,
85 and the sgACC, implicated in the modulation of automatic emotional behavior,
86 are additional paralimbic regions that require further investigation. Initial findings, nonetheless, position the pACC and related networks as mediators of impaired affect-regulation and cognitive processes in patients with FND.
Several important implications arise from the proposed neurobiological framework. The outlined cortico-cortical and cortical-subcortical pathways allow clinicians to understand the presentation of functional symptoms independent from notions of psychic tension and the unconscious mind; functional deficits in-and-of-themselves may not necessarily be symbolic of the particular emotional stress experienced by the patient, but rather may be the product of intrinsic neural connectivity patterns. Thus, the basic form or phenotype in which functional symptoms present may be driven by intrinsic neural connections, whereas the specific context of functional symptoms (i.e., why and when symptoms occur) may be a product of interactions between the patients’ psychosocial stressors and attentional, limbic/paralimbic, and sensory-motor cortices.
Also, neurologists are frequently frustrated by interactions with FND patients, since clinicians may view them as fabricating their symptoms. This frustration is markedly less when treating unawareness in right-hemisphere stroke patients with neglect. Conceptualizing unilateral functional motor and somatosensory deficits as functional neglect or unawareness, rather than willful deception or simulation, may improve the therapeutic alliance between physician and patient, and potentially increase the number of clinicians inclined to work longitudinally with FND patients. Importantly, this framework does not, in our opinion, undervalue the role of affective disturbances in the presentation of functional neurologic symptoms, but rather contextualizes the patients’ presentation in terms readily understood by clinicians. Finally, the identification of cortical sites, including the PPC and the dorsolateral PFC, offers targets to investigate for their therapeutic intervention in patients with FND; these include the potential use of neuromodulation techniques (i.e., transcranial magnetic stimulation) to modify aberrant functional activation patterns.
Limitations and Other Considerations
There are several important limitations to address regarding the suggested conceptual framework. The discussion is limited to unilateral functional motor and somatosensory disturbances and does not incorporate other FND subtypes, including astasia-abasia (functional gait disorder),
94 functional blindness,
95 and psychogenic non-epileptic seizures. All FND subtypes may not have the same mechanistic network explanations for distinct phenotypes, and research in these and other subtypes warrant further investigation. Also, many of the functional neuroimaging studies cited examine single cases
18,26,31 and case–control studies with a small number of subjects,
20,24,25 increasing the possibility of type I statistical errors. Negative affective disturbances were also not consistently controlled for methodologically across studies, suggesting potential confounds for the prefrontal, subcortical, and limbic findings.
Comparisons with deliberate feigning or hypnosis-induced functional disturbances are also omitted. Although deliberate feigning has been used as a comparison condition in some FND neuroimaging studies,
19,20,96 the neural correlates of feigning are not yet well understood, which adds increased difficulty when comparing the neural activation patterns to patients with FND. Hypnosis-related research is also not incorporated in the FND framework, since hypnosis requires more exploration, but we acknowledge the possibility of an overlap in the biology of hypnosis and FND.
97The functional-unawareness concept may also be interpreted with evolving psychological models. It is important, however, first to note that, when considering the integration of psychological and neural models, researchers should not necessarily search for the neural correlates of a given psychological concept, which may or may not be supported by empirical evidence. With this in consideration, one possible psychodynamic formulation invokes mechanisms of adaptive and maladaptive psychological defense.
98 Defense mechanisms are conceptualized as the ego’s efforts to cope with psychological stress, and pathologic somatic-based defenses thought to be used by patients with FND would reduce transpsychic conflict through bodily displacement. The ACC has been implicated in emotional expression/regulation, maladaptive neuroplasticity, and conflict monitoring (cognitive neuroscience definition), and offers intriguing possibilities for integrative synthesis. However, more research is needed to fully integrate psychological and neurobiological concepts in patients with FND.
Conceptualization of FND as a disorder of unawareness also does not explain differences between hypokinetic and hyperkinetic functional motor symptoms. Inhibitory or disinhibitory effects of premotor regions (i.e., SMA) on primary motor cortices, and modulated by interactions with the PPC, dorsolateral PFC, and limbic/paralimbic regions, may help clarify phenotypic differences.
27,34 This may be analogous to differences in alien hand syndrome phenotypes; medial-frontal lesions result in hypermotor, foreign behaviors, whereas posterior variants present more commonly with disturbances of posture and levitation.
99 Also, further clarification is needed of the role of the left hemisphere in unawareness, as seen infrequently, for example, in left-hemisphere lesions resulting in contralesional visual-spatial neglect or unawareness of deficit in Wernicke’s aphasia.
36,37 Lastly, this article reconciles many of the distinct and overlapping neural-circuit findings across paradigms in FND, but falls short of providing a unifying neurobiological model incorporating the entire body of unilateral motor and somatosensory FND literature.
96,100 More detailed understanding will require larger subject groups, multiparadigm neuroimaging studies (including neuroimaging at rest to explore default-mode network disturbances), and non-imaging, cross-disciplinary research to provide a fully integrative model.