The Orbitofrontal Cortex and Related Structures
The OFC is critical for decisions dependent on incentive gain as well as the emotional experience associated with outcomes.
2 The OFC is composed of four cyto-architectonic areas: Brodmann area (BA)11, anteriorly; BA13, posteriorly; BA14, medially; and BA47/12, laterally.
3 , 4 The lateral OFC (area 47/12) receives and integrates visual information from the inferior temporal cortex, auditory information from secondary and tertiary auditory areas, somatosensory information from the secondary somatosensory and parietal cortex, and heteromodal inputs from the superior temporal cortex.
5 The orbitofrontal cortex is functionally organized such that the medial portion monitors and decodes
rewards, whereas the lateral portion evaluates
punishment.
6 Reward value for concrete, primary reinforcing factors, such as touch and taste, are encoded in the posterior OFC, whereas the value of more-complex, secondary reinforcing factors, such as money, are encoded in the anterior OFC.
7The OFC is located adjacent to the anterior cingulate cortex (ACC) and the frontal polar cortex;
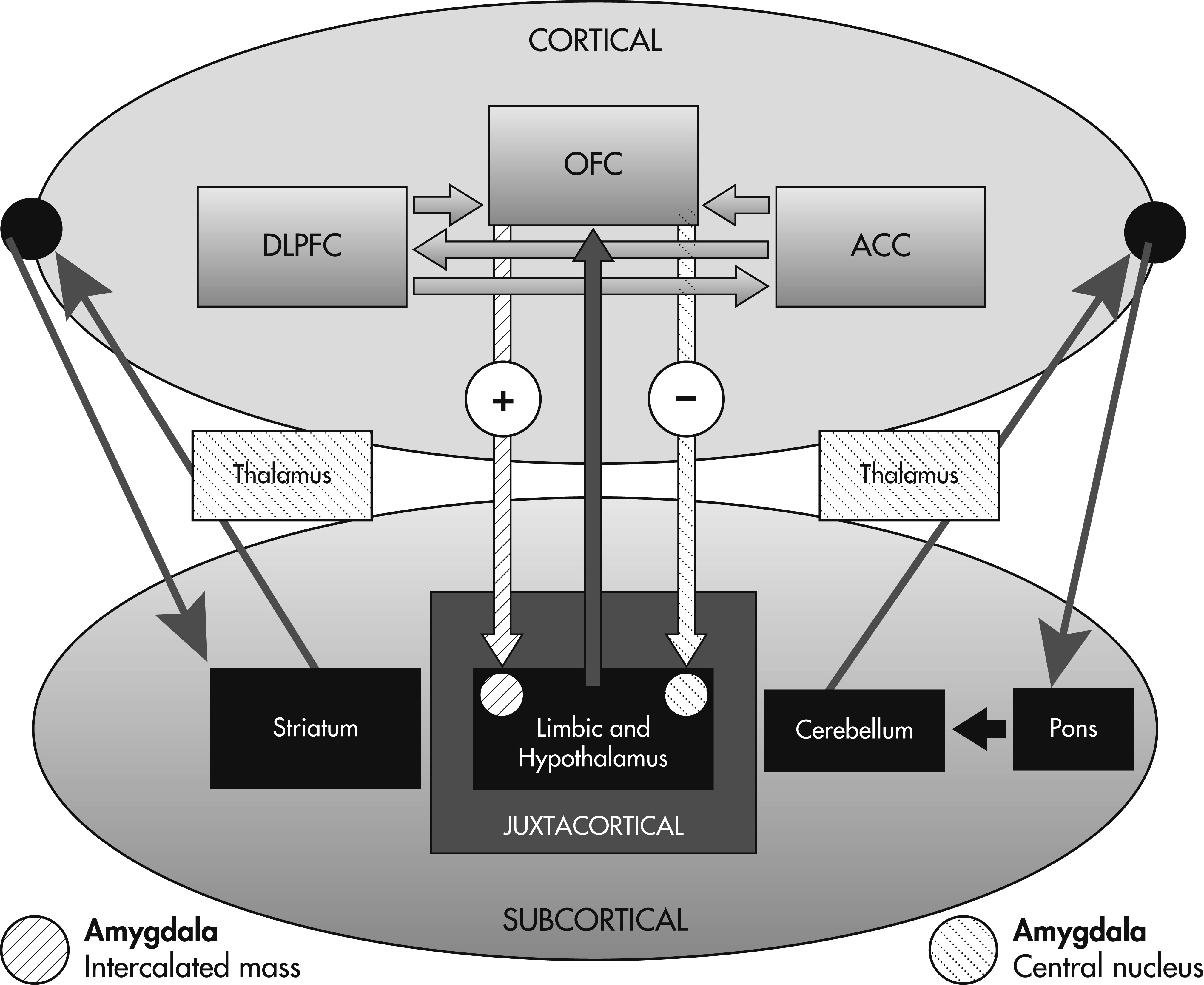
8 these three regions are jointly referred to as the ventromedial prefrontal cortex (VMPC) although the structures designated by this region may vary according to the literature. The OFC has cortico–cortical connections with other frontal structures involved in decision-making, including the DLPFC and ACC. Furthermore, the OFC is distinguished by strong bidirectional links with the anterior cingulate, insular, and medial temporal cortices, and with the amygdala and hippocampus.
9 Networks between the OFC, temporal association cortex, amygdala, and lateral prefrontal cortex are largely responsible for emotional processing.
9The temporal cortex contains feed-forward projections to the amygdala, which has bidirectional, partially segregated, and highly specific connections with the OFC.
10 The OFC has two distinct efferent projections to the amygdala, resulting in separate outcomes: 1) projections to the intercalated mass of the amygdala lead to disinhibition of autonomic structures within the hypothalamus (autonomic arousal); and 2) projections to the central nucleus of the amygdala lead to inhibition of hypothalamic structures (autonomic homeostasis).
10Patients with OFC lesions suffer from two types of deficits that ultimately affect decision-making. First, individuals fail to shift stimulus–response contingencies and suffer from impaired reward-processing. In other words, patients may fail to alter their decisions to a given stimulus despite a negative associated outcome. Second, patients are deficient in tasks requiring empathy or theory of mind (TOM), failing to process and recognize the emotions of others, and manifest impaired judgment in social contexts. Such limitations in social learning and emotional identification frequently lead to a clinical syndrome consisting of disinhibition, impulsivity, increased risk-taking behavior, and an inability to alter one’s behavior despite negative social consequences.
Evidence for the role of the OFC in emotion-based reward-processing comes from nonhuman primate investigations, human lesion studies, and neuroimaging. On a cellular level, OFC neurons fire during reward contingencies, particularly when monkeys anticipate a large reward as opposed to a small one.
11 On the other hand, OFC neurons manifest a decreased firing rate when choices predict larger punishment.
12 The firing of OFC neurons can further be influenced by whether or not the stimulus was presented previously, and by the immediacy of the reward.
13Previous investigators have studied the role of the OFC in human decision-making by administering a gambling task in frontal-lesion patients. Studies using the Iowa Gambling Task (IGT), an assessment of decision-making under varying conditions of risk, first established that patients with OFC lesions suffer from impaired decision-making.
14 Also, the Cambridge Gambling Task (CGT), another test of decision-making, minimizes the amount of required learning and retrieval that may involve other brain regions, such as the DLPFC.
15 Both tests address risk-taking behavior as manifested by an ability to moderate one’s betting strategies on the basis of experience (i.e., normal subjects would avoid placing large bets when the odds of winning are low). Although these studies have provided valuable insights into the role of these brain structures, they are also limited by their inclusion of subjects with diffuse frontal lesions (tumor, infarct, aneurysm, traumatic brain injury, and surgery for intractable epilepsy) that extend beyond the ventromedial prefrontal cortex into the dorsomedial PFC, dorsolateral PFC, temporal poles, and the basal forebrain.
Decision-making in VMPC patients is characterized by increased risk-taking behavior. Compared with normal and brain-damaged control subjects, VMPC patients have an increased predilection for choosing cards from disadvantageous decks during the IGT.
16 A study of 11 subjects with chronic frontal-lobe injuries showed that patients with focal left-sided OFC and ventrolateral prefrontal cortex lesions were more likely to exhibit increased risk-taking behavior (rather than impulsivity) on a gambling task, as compared with patients with bilateral frontal and non-frontal injuries.
17 VMPC patients also suffer from sympathetic dysfunction when confronted with high-stakes decisions. Over the course of the IGT, normal patients with risky decks develop an anticipatory skin conductance response (SCRs) 5 seconds before making card choices. Such anticipatory SCRs are absent in patients with VMPC lesions.
18The poor decision-making in this population is further characterized by an inability to
adjust stimulus–reward contingencies. Patients with OFC lesions fail to modify their gambling strategy on the basis of feedback and continue to make the same gambling mistakes, ending up with greater net losses than control subjects. The mechanism behind recurrent maladaptive decisions in OFC patients may be deficient counterfactual thinking (comparing “what is” with “what could have been”), an important component of regret and subsequent learning. Also, these patients fail to experience negative emotions, despite being informed that they had suffered a gambling loss.
19Patients with VMPC lesions further exhibit deficient emotional processing: they fail to experience and recognize social emotions (compassion, shame, guilt) and possess a low threshold for anger and frustration.
20 , 21 Despite preserved recognition of facial expression, OFC patients rate faces displaying negative emotional expressions as more approachable, compared with subjects with OFC-sparing frontal lesions and normal-control subjects.
22Such impairments in emotional processing may translate to patients’ taking an impersonal approach toward moral decision-making. One study subjected 6 bilateral VMPC patients, 12 brain-damaged controls with lesions sparing the VMPC, and 12 normal controls to high-conflict moral dilemmas that required balancing group welfare with the aversion of harming another individual (e.g., smothering one’s baby to save a group of people).
23 Patients with VMPC lesions were more likely than both brain-damaged controls (lesions sparing VMPC) and normal-controls to endorse actions that resulted in harming an individual to save a group. Also, VMPC patients showed lower empathy, embarrassment, and guilt scores, according to the Iowa Rating Scare of Personality Change. The relevance of the VMPC in decision-making has further been confirmed by functional magnetic resonance imaging (fMRI) studies demonstrating activation of the VMPC with tasks requiring moral judgments; passive viewing of morally salient photos; and the elicitation of charity, fairness, or guilt.
24 – 26The clinical and experimental observations described above have led to the proposition of a VMPC-based neuro-moral network.
27 This network may be functionally lateralized to the right hemisphere. Patients with right-sided, as opposed to left-sided, lesions of the VMPC, exhibit impaired life decision-making ability, as reflected by the maintenance of gainful employment, as well as IGT/CGT performance.
16 The right VMPC is also activated with performance of the CGT on PET imaging.
28Although the role of the VMPC in emotional and reward processing has been extensively described in the literature, the OFC has connections with both the anterior insula and limbic system (hippocampus, amygdala) that are critical to the decision-making process. The amygdala, a structure activated by decreased perceived trustworthiness, facilitates social judgments about trusting others.
29 Recent work has shown that the anterior insular cortex, a multifunctional structure involved in perceptual functions, speech, sensorimotor integration, body awareness, and emotional decision-making,
30 serves a similar role in decision-making as the OFC. With connections to the ACC and VMPC, the anterior insula integrates autonomic information with emotional and motivational functions. Patients with insular lesions demonstrate similar impairments on the IGT as OFC patients, but these studies were limited in that subjects’ lesions extended into limbic and ventromedial prefrontal regions.
31 Furthermore, patients with insular lesions have been described as demonstrating an “emotional bluntness toward risk.”
32The Dorsolateral Prefrontal Cortex (DLPFC) and Anterior Cingulate Cortex (ACC)
Decisions dependent on emotions and stimulus–response contingencies predominantly activate the OFC, but this structure interacts with other frontal circuits, such as the DLPFC and the ACC, through cortico–cortical connections, to facilitate decision-making on various levels.
33The DLPFC (BA 9 and 46) occupies the superior and lateral regions of the frontal lobes and is organized along a dorsal–ventral axis, with the dorsal DLPFC monitoring working memory and the ventral DLPFC encoding and retrieving information stored in posterior cortical-association regions.
2 , 34 Similar to the OFC, the DLPFC has strong limbic connections as well as cortico–cortical connections throughout the temporal, parietal, and occipital cortices.
34 , 35Lesions of the DLPFC result in a frontal dysexecutive syndrome, in which patients exhibit impairments in planning, inhibitory control, strategy development, cognitive flexibility, and working memory.
36 Goel and Grafman
37 describe the case of “PF,” a previously successful architect who underwent surgery to remove a meningioma in the right DLPFC, and subsequently developed impaired problem-solving ability at work. Functional imaging studies show that the DLPFC is activated during impersonal dilemmas, suggesting a more calculated, emotionless approach toward decision-making.
38 , 39 The DLPFC also appears to play an important role in legal decision-making. An fMRI study of 16 normal subjects, given scenarios describing varying degrees of responsibility for an act by a protagonist, showed increased right DLPFC activation when subjects assigned blame, whereas increased amygdala, medial PFC, and posterior cingulate cortex activity was associated with the magnitude of determined punishment.
40The ACC occupies the medial portion of the prefrontal cortex. This region has cortico–cortical connections to the OFC and DLPFC, as well as subcortical projections to the nucleus accumbens. The most anterior portion of the ACC, BA 25, has been implicated in depression,
41 and may participate in linking decision-making to emotional tone.
42 The ACC’s role in decision-making may be related to the modulation of other select prefrontal regions, such as the OFC and DLPFC. Studies have shown that particularly complex decisions involve the ACC, along with multiple brain structures, such as the OFC, DLPFC, and insular cortex.
43 , 44 Furthermore, patients with bilateral OFC lesions with involvement of the ACC performed worse on tasks requiring evaluation and recognition of social cues than OFC subjects without ACC involvement.
45The ACC is recruited for highly ambiguous choices, such as in situations in which there are conflicting options and a high likelihood of error. Single-unit recording studies of cell populations in the macaque dorsal ACC during the performance of a reward-based decision task (choosing whether to push versus turn a handle for reward), have shown that these cells are five times more active during the interval between reward-reduction and the initiation of the newly-corrected movement.
46 Also, the ACC participates in performance-optimization and evaluation by using previous reward experiences to guide choices. A case study of a patient with cortical dysplasia affecting the mediofrontal cortex demonstrated selective impairments in ambiguous decision-making pre- and post-surgically.
47 fMRI studies have revealed ACC activation during periods of reduced reward or upon receiving negative feedback for incorrect answers.
48A Model for Decision-Making
The decision-making process depends on interactions between the three prefrontal regions described above with subcortical structures that include the limbic system, basal ganglia, thalamus, cerebellum, and pons. In contrast to memory
71 and language, a formal decision-making network has yet to be defined. Wallis has assigned the OFC, DLPFC, and ACC specific roles in the process of decision-making.
13 According to this model, the OFC, through connections with the amygdala and limbic system, encodes the value of a reward outcome in relationship to a particular decision. The DLPFC then processes and utilizes this information in a top-down fashion to construct a specific plan for that reward outcome based on the OFC’s appraisal of reward value. Finally, the ACC serves to evaluate the likelihood of success for the plans generated by the DLPFC before the execution of the behavioral response. This model provides a rationale for previous observations that patients with extensive prefrontal lesions extending beyond the OFC perform worse on decision-making tasks such as the IGT.
Graybiel has proposed that components of the neocortical–basal ganglia loops are essential for learned actions to become habitual, and that abnormal activity within these loops is implicated in a range of clinical disorders related to action-compulsion, as occurs in obsessive-compulsive disorders and addiction behaviors.
72 Inhibitory prefrontal projections to the basal ganglia may serve a self-regulating purpose, providing signals that direct behaviors away from immediate and toward distant rewards.
13 In general, PFC activity is associated with long-term outcomes, whereas subcortical activity is associated with more immediate outcomes.
Heatherton and colleagues describe the prefrontal cortex as providing top-down control affecting the striatum and limbic cortices. The nucleus accumbens (NA) is a dopamine-activated region located within the striatum responsible for mediation of immediate reward, and it has been implicated in addiction. Rats with excitotoxic lesions involving this region demonstrate an increased predilection for immediate rewards and show difficulty learning instrumental responses using delayed reinforcement.
73 The NA receives dopamine inputs from the ventral tegmental area (VTA) within the brainstem, and disruptions to this neurotransmitter’s activity may contribute to impulsive decision-making, as evidenced by disease states that include schizophrenia, amphetamine abuse, and Parkinson’s disease, particularly in the setting of dopamine agonists.
74 The NA receives inhibitory inputs from the medial prefrontal cortex; cocaine users or smokers asked to inhibit their cravings to environmental cues manifest increased prefrontal activity on functional imaging.
75 The prefrontal cortex also has inhibitory connections with the amygdala and limbic system (i.e., OFC, with inhibitory connection to the central nucleus of the amygdala), and compromise of this pathway has been implicated in mood disorders without obvious structural abnormalities. This model is supported by an FDG-PET study showing frontal hypoperfusion and limbic hyperperfusion in subjects classified as “affective murderers.”
76 Ultimately, inhibitory projections between the prefrontal cortex and subcortical regions (striatum and limbic system) provide a balance between self-control and reward incentives, emotions, or attitudes.
The decision-making process depends on cortico–cortical and cortico–subcortical interactions such that all three prefrontal regions (OFC, ACC, and DLPFC) may be defined by the pattern of their connections. In other words, decision-making impairment may result from lesions not only involving the cortical regions, but also the limbic system, subcortical nuclei and white matter. The cortex is distinguished in that it maintains direct connections through associational fibers with other neocortical regions as well as the subcortical gray matter and limbic cortex.
On the basis of the prefrontal circuits and models described above, we conceptualize a novel decision-making network depicting the projections between neocortical and subcortical regions (
Figure 1).