The Medial Forebrain Bundle: A Ponto-Frontal Projection Pathway
Textbooks of neuroanatomy typically describe the fronto-pontine tract (Arnold’s bundle) as part of the anterior limb of the internal capsule (ALIC) and as the most anterior and medial fiber structure at the level of the crus cerebri, directly adjacent to the corticospinal tract.
13 These classical descriptions focus predominantly on the topography of the anterior limb of the internal capsules and are supported by more recent studies that apply polarized light to identify fiber-tract directions in the anterior limb of the internal capsule and the brainstem on the basis of histological slice sections.
27–31 However, whether Arnold’s bundle can be found in humans is questioned in the literature, and some authors deny its existence.
32 Postmortem examinations of lesioned brains from the frontal-leucotomy era
33,34 have demonstrated the existence of descending or ascending tracts that most likely represents Arnold’s bundle in humans. According to these studies, Arnold's bundle originates from the dorsal (superior) and lateral convexity of the prefrontal cortex,
20 and not from the agranular parts or the OFC.
However, a recent MRI study in patients after anterior capsulotomy has identified an apparent reciprocal corticopetal pontofrontal projection.
21 Classical neuroanatomy does not clearly reflect on a possible bidirectionality of the fronto-pontile projection system (Arnold’s bundle). In this sense, this study extends neuroanatomical knowledge by adding a fiber pathway that is antidromic to Arnold’s bundle. These authors inferred this projection on the basis of early post-axotomy accumulation of axonal lipoproteins that could be identified by MRI. More specifically, accumulation of protein was found in the upper midbrain after lesioning fibers in the anterior capsule in the frontal lobe thus further downstream. This indicates that the cell bodies of this neuronal pathway are situated close to the midbrain (and not in the frontal lobe), indicating a mesencephalo-frontal trajectory. The projection proposed by Hurwitz is clearly extending the classical neuroanatomical description and has not yet been included into newer texts on the neuroanatomy of psychiatry.
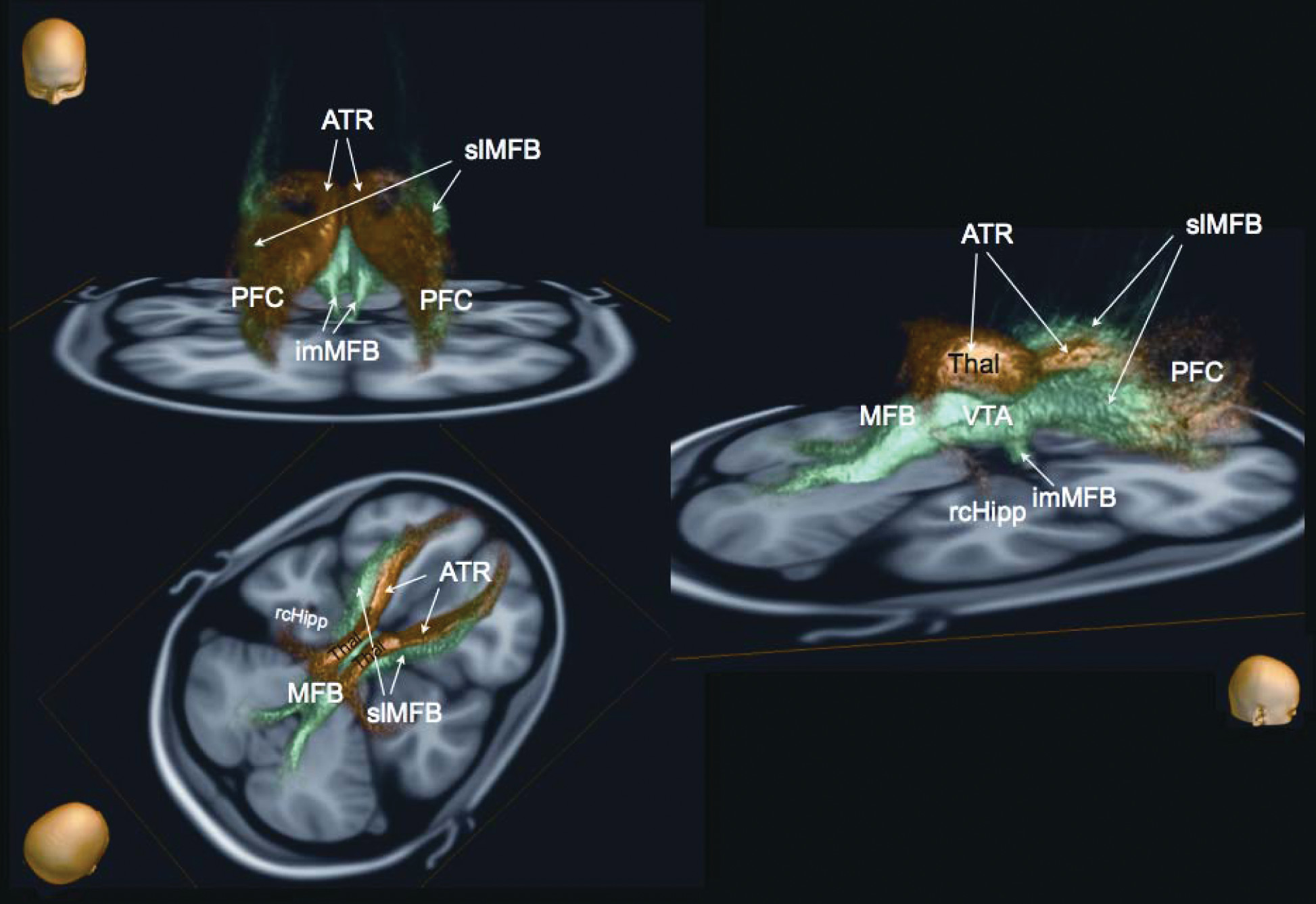
3,14,35–38 Thus, Hurwitz and colleagues first described a reciprocal mesencephalo-frontal pathway in the living human brain. This pontofrontal projection appears to coincide with the supero-lateral branch of the MFB (slMFB) described in our earlier work.
1 These results have recently also been replicated by others using the DTI technique in a context of non-motor projections of the cerebellum.
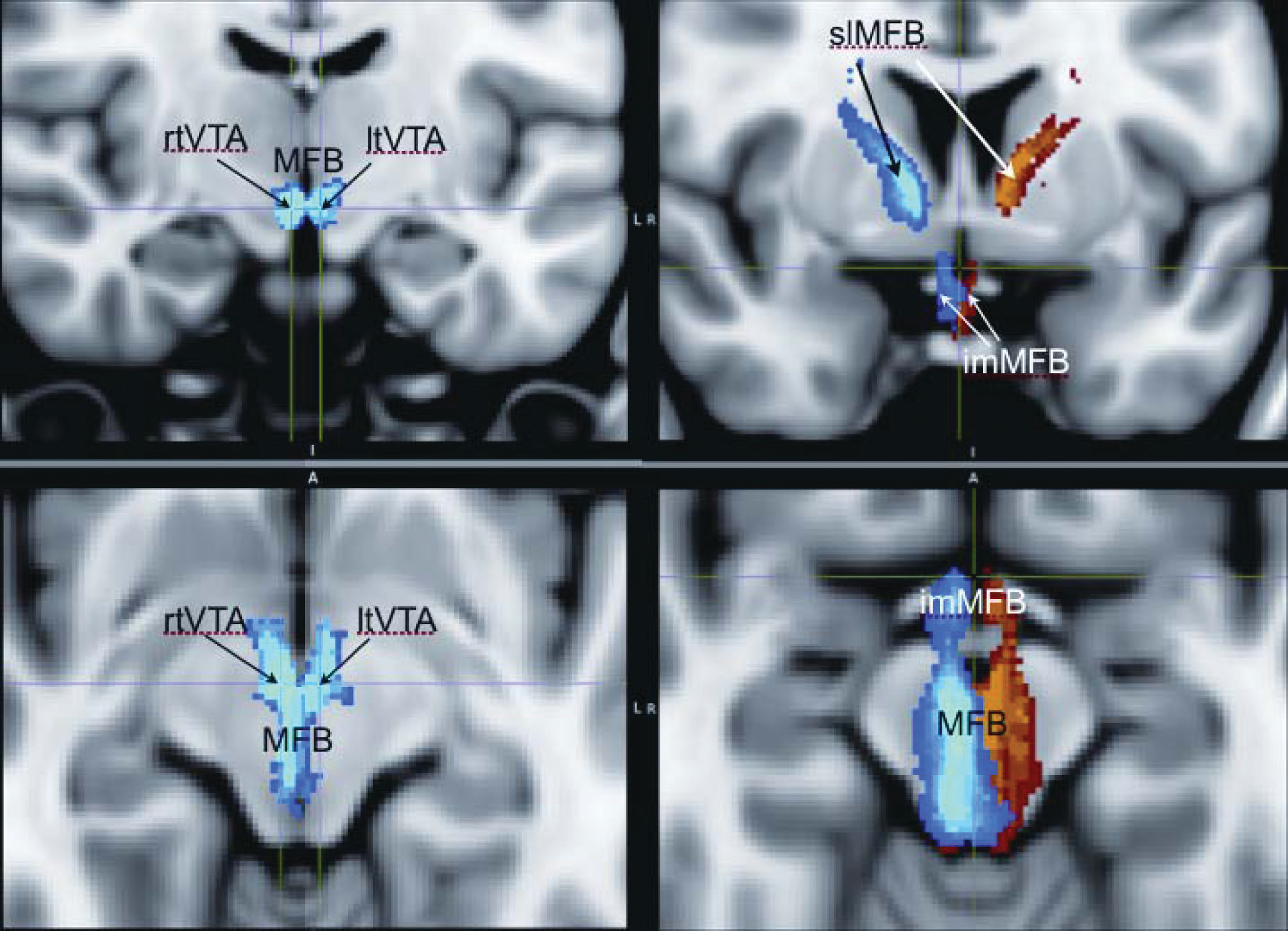
59A Newly-Described Supero-Lateral Branch of the MFB (slMFB)
Anatomically, the MFB in all species that have been studied connects the ventral tegmental area (VTA) with the lateral hypothalamus (LH) and the nucleus accumbens (NAcc);
13,19,39–42 Le Gros Clark noted that this pathway also connects to the olfactory bulb.
13 Nauta had widened the limbic system concept with what he defined as the limbic midbrain. According to his description, the dopamine-containing VTA neurons interface directly with the limbic forebrain via the perifornical region.
43 Experimental findings from rodent and primate studies in the last quarter-century list several more pathways of the VTA with basal forebrain structures. These targets are interconnected via the MFB, which serves as the major route of passage along the lateral hypothalamus, including connections to the medial hypothalamus, sublenticular region, lateral and medial preoptic region, diagonal band, septal nuclei, ventral pallidum, and ventral parts of the bed nucleus of the stria terminalis.
37In humans, this pattern of mesolimbic network remains to be described in the classical context of anatomical textbooks. Because of the distinct phylogenetic developmental growth of the brain, the MFB has to follow somewhat distinct trajectories to link up with the aforementioned key limbic structures in man.
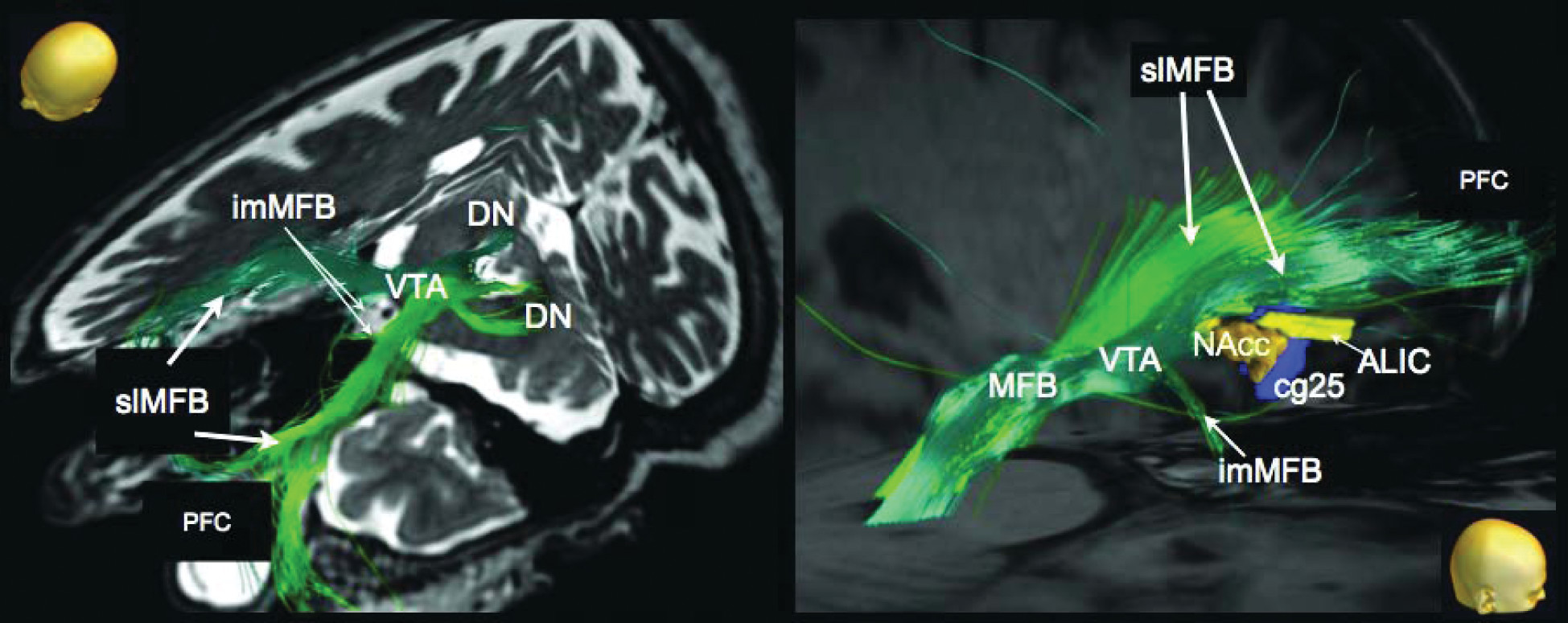
1 The slMFB described here is ideally placed to serve this role. The slMFB arises out of the trunk of the MFB, and its links with the VTA, NAcc, and ALIC can be well characterized (
Figure 3). Our data thus enhance, rather than contradict, established neuroanatomical knowledge in other species.
Clinical Significance
Stereotactic targets in deep brain stimulation (DBS) used for treatment-resistant psychiatric disorders have included cg25 (in depression)
44 and, more recently, nucleus accumbens septi (NAcc)
9, 45 as well as the anterior limb of the internal capsule (ALIC)
20, 46 for depression, and obsessive-compulsive disorder (OCD). The exact neuroanatomical locations for optimal therapeutic effects and the precise mechanism of DBS in these clinical conditions remain unclear,
2,44,45 but may include both suppression and activation of neural tissues. For ALIC- and NAcc-DBS, one possible explanation for psychobehavioral benefit is activation of the slMFB,
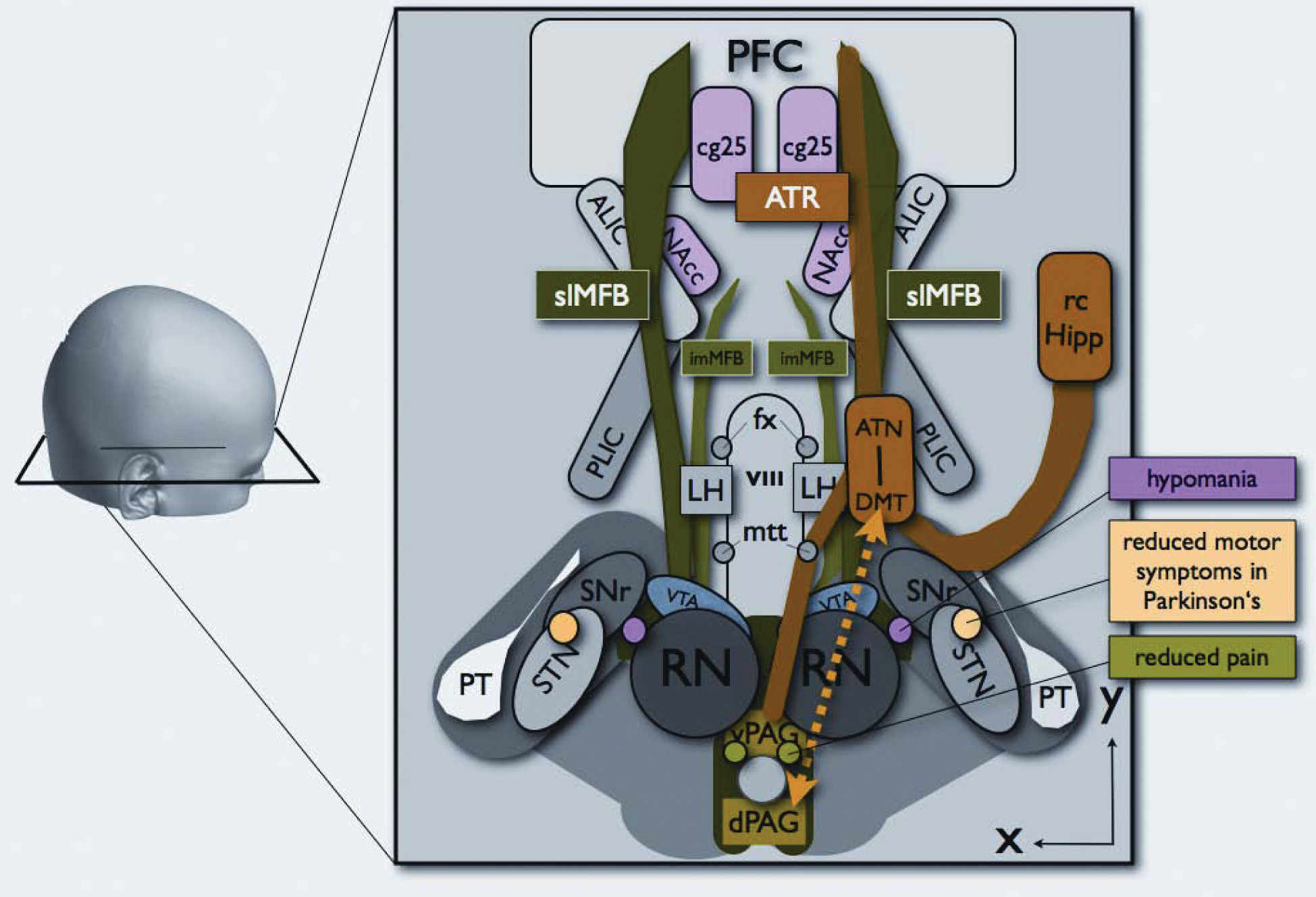
2 which connects to critical higher limbic targets. Stimulation of this system—traditionally called “the brain reward system” —would result in an antidysphoric effect by galvanizing the appetitive motivational SEEKING system that promotes euphoria, accompanied by a motorically-activated, psychologically-energized, and emotionally highly-motivated foraging-planning state.
3 (In fact, the brain has several reward systems.
11,12) Considering these affective changes, over-activity of the slMFB may promote manic states,
1 and, hence, also mood switching in bipolar disorders, whereas the pattern of psychopathology of an expansive mood, enhanced energy, and psychomotor acceleration is consistent with an over-functioning SEEKING system. Such an explanation could also account for the hypomanic states that may complicate DBS subthalamic nucleus (STN) stimulation for Parkinson’s disease, where an incorrectly-placed electrode may inadvertently activate this system.
1 Finally, under-functioning of this system could lead to depressive fatigue states, and, as such, suggest a target for future clinical research, given the prevalence of debilitating fatigue syndromes and the limited efficacy of currently available therapies. Indeed, two reported patients treated with anterior capsultomy for depression and OCD developed a severe fatigue syndrome postoperatively. We hypothesize that surgery in those cases might have also severed parts of the slMFB that, as we have shown, lies in close proximity to the ATR in the area of ALIC.
The ATR, in contrast to the MFB, may play a role in depressive illness via the PANIC emotional system that has been revealed by mapping separation-distress calls.
11,12 Such brain regions are aroused when humans experience sadness. This is hypothesized to occur because of its presumed connections with cg25, which has been shown to be overactive in depressed patients.
47 The horizontal fibers of the ATR are directed to the anterior pole of the frontal lobe, which includes the rostral anterior cingulate gyrus and orbitofrontal cortex.
Earlier work of Herman and Panksepp mapped out the separation-call in guinea pigs, which is directly modulated by endogenous opioids.
60 In a human subject, only recently, during a case of anterior thalamic nucleus stimulation for intractable epilepsy, our group was able to repetitively induce acute episodes of depression through activation of deeper electrode contacts located in the ATR (unpublished data).
In rodents, stimulation of several forebrain and anterior diencephalic regions (dorsomedial thalamus, ventral septum, dorsal preoptic area, and bed nucleus of stria terminalis, as part of the extended amygdala) can elicit separation distress. In the light of these facts, it could also be questioned whether the MFB only conveys euphoria. However, with regard to the theorizing of MacLean, it would be reasonable to consider that primary and secondary affect-processing mandates connections of the medial forebrain bundle and anterior thalamic radiation other than the prefrontal cortex. A potential explanation is that ATR and MFB connect at these basal forebrain regions.
7,12,61 The relationship of the MFB SEEKING system to these specific areas is not known. It suggests possible areas of interaction that should be investigated in the future.
Deep brain stimulation of the white-matter tracts adjacent to the subgenual cingulate gyrus has been demonstrated to normalize (reduce) pathological hyperactivity of the subgenual cingulate gyrus and adjacent orbitofrontal cortex and is associated with sustained improvement in patients with treatment-resistant depression.
21,44 Thus, the MFB and ATR mediate very different, and, possibly, opposing, affective states. As suggested by our in-vivo tracking results, both are located in close proximity within ALIC but are now clearly separable anatomical and, likely, also functional structures. They provide candidate networks for the promotion of opposing emotional states and therefore may be critical for affective equilibrium and disequilibrium.
The anterior thalamic radiation is a structure that has long been targeted for lesioning or DBS in the treatment of psychiatric disorders.
2,3,10,21,31,48,49 Why other groups that have applied fiber-tracking in the same brain areas did not describe or identify the slMFB as an individual structure is unclear. We believe that those fibers that we associate with the slMFB have also been seen by other groups, but perhaps were allocated to other tracts.
10,22–25,50 Indeed, Wakana et al. describe a tracking procedure for the ATR starting with a seed region (ROI) from the frontal lobe that covers the anterior limb of the internal capsule and showed connections to the brain stem that they regarded as atypical for ATR.
51 We infer that their ATR has included the slMFB because of the close proximity of slMFB and ATR to their tracking ROI.
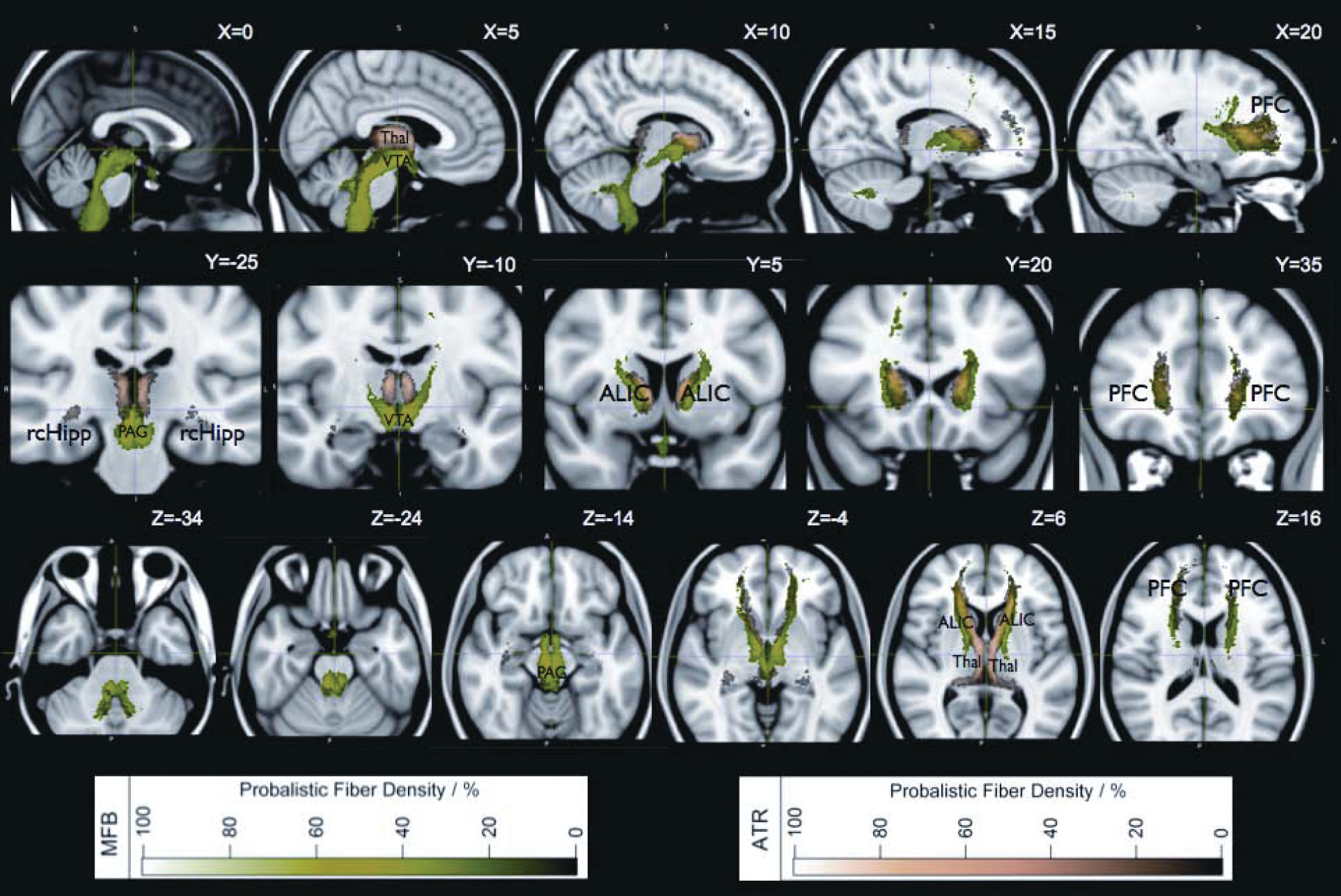
Figure 5 explains how the ICBM ATR template from the Johns Hopkins University JHU White Matter Tractography Atlas (ATRj)
58 might in reality be a combination of ATR and slMFB. The 28 subjects studied for the Johns Hopkins’ white-matter atlas compared with our 16 patients led to quite similar statistical variance of tracking results if one considers that the ATRj indeed composes a superposition of our ATR and slMFB. For a more thorough comparison, refer to
Figure 5 and Supplement
Figure 2.