Autism is characterized by impairments in social interaction, and verbal as well as nonverbal communication skills, and a restricted and odd range of interests and activities, with onset before 3 years of age.
1 Since its first description in 1943,
2 the neurobiological mechanisms underlying the behavioral characteristics of autism have remained incompletely understood.
The serotonergic (5-HT) system, a focus of interest because of its role in emotional behavior, has been implicated in autism. A number of atypical findings in emotional performance are observed in autism, including decreased emotional memory.
3 A significant proportion of autistic individuals tend to demonstrate elevated levels of serotonin in their peripheral blood.
4–7 This serotonin increase has not been found in the cerebrospinal fluid (CSF), where the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) has been measured in autistic individuals.
8 However, an atypical blood platelet response to serotonin is observed in autism.
5–7 Specifically, abnormal activity of 5-HT
2 receptors has been reported in circulating platelets of autistic individuals.
7 It has been speculated that related abnormalities in this receptor subtype may exist elsewhere in the body in autism. Evidence suggesting decreased central 5-HT responsivity to neuroendocrine challenge has been observed in autism.
9 Early abnormalities in the serotonergic system may also have significant impact on the development of the brain in autism, due to the action of serotonin as a growth factor in embryogenesis.
10Recent studies have begun to explore serotonin changes in the brain in autism. Regional alterations in serotonin-synthesis capacity have been reported in the brain in a small series of autistic individuals, particularly in the left thalamus and frontal lobe in male subjects.
11 Serotonin transporter-binding has also been found to be decreased in autism.
12 Others, using a single photon emission computer tomography (SPECT) ligand,
13 have reported decreased frontal, temporal, and parietal 5-HT
2 binding in autism spectrum disorder (ASD). One more-recent report, which used positron emission tomography (PET) to examine 5-HT2 binding in family members of patients with ASD, revealed an overall decrease in binding of [
18F]setoperone, along with specific decreases in cortical regions, for example, frontal, parietal, temporal, and occipital areas.
14 Our purpose is to examine these areas reported for family members with [
18F]setoperone in autism patients, while also examining the subcortical regions, that is, thalamic regions reported by Chugani et al.
11We wished to determine whether abnormalities in 5-HT
2 receptor binding might exist in frontal and thalamic regions, particularly on the left, where unilateral regional alterations in brain serotonin-synthesis capacity have been previously described in a small series of autistic individuals.
11 Further interest in frontal lobe 5-HT
2 binding and, by its reciprocal connectivity, the thalamus, is raised by the regional abnormalities in metabolism that have been observed in the orbitofrontal cortex in obsessive-compulsive disorders.
15 We are also interested in the temporal lobe because of the presence of neuroanatomical abnormalities in medial temporal structures in autism.
16 Furthermore, autism is commonly associated with anxiety, obsessiveness, and atypical responses to emotional stimuli, all potentially related to 5-HT function,
17–20 and regional abnormalities in 5-HT transporter binding have been observed in the temporal lobe in panic disorder.
21 These findings also lead to the question of whether there is atypical temporal 5-HT
2 binding in autism. Serotonin is also known to be critical for long-term potentiation (LTP) in the hippocampus,
10 which might relate to aspects of memory performance observed in autism.
The serotonin ligand, [
18F]setoperone, was used to visualize 5-HT
2 receptors in these regions of interest through PET. This compound is a highly specific to 5-HT
2 receptors, with a binding affinity 25–50 times higher than those for dopaminergic D2 or adrenergic α1 or α2 receptors, and far greater than those of histaminergic H1 or μ-opiate binding,
22 and it was utilized in the previous study examining family members of patients with ASD.
14 Receptor binding was compared across regions of interest between age matched autistic and control subjects.
Method
Eight high-functioning adults with autism (5 men, 3 women) and 12 non-autistic adults (8 men, 4 women) without any other known neurological or psychiatric condition independent from autism were initially imaged. Groups were matched for age (autism: 31.0 (SD [standard deviation]: 8.0 years old; control: 31.8 [9.8] years old;
t[18]=0.18; NS) and full-scale IQ (autism: 114.0 [14.7]; control (when available): 122.3 [6.8];
t[10]=1.03; NS). The diagnosis was confirmed by means of the Autism Diagnostic Interview–Revised (ADI–R)
23 in seven of the participants, and one by DSM-IV criteria,
1 since no parent was available for ADI–R interview. All subjects were at least 18 years of age and had a full scale IQ of at least 85. All women were confirmed as not-pregnant by urine screening. Subjects were asked to refrain from consuming chocolate, turkey, pineapple, or bananas for 1 day before imaging, and were asked not to consume an unusual amount of milk, in order to avoid dietary changes that might affect peripheral or central serotonin. Medication lists were also reviewed for serotonergic agents (one subject from each group was subsequently found to be taking a longstanding, stable dose of a serotonin-selective reuptake inhibitor (SSRI), and they were excluded). Autism subjects were voluntarily recruited primarily from the Boston-area clinic populations. Control subjects were also voluntarily recruited from the Boston area. All subjects consented in accordance with the Institutional Review Board of the Massachusetts General Hospital.
Images were acquired with a PC-4096 PET camera (Scanditronix AB; Uppsala, Sweden). The primary imaging parameters of the PC-4096 camera are in-plane and axial resolution of 6.0 mm FWHM and 15 contiguous slices of 6.5 mm. PC4096 camera data were acquired in 2D mode and reconstructed using a conventional filtered back-projection algorithm to an in-plane resolution of 6.0 mm FWHM. Photon-attenuation measurements were made with rotating pin sources containing 68Ge. Corrections were made for scattered radiation, random coincidences, and counting losses resulting from dead time in camera electronics.
After placement of a venous catheter in the right arm, subjects were positioned supine on the imaging bed with arms extended out of the field of view and head immobilized with individualized head-holders. As in previous studies,
24,25 approximately 7mCi of [
18F]setoperone (at maximal specific activity) was bolus-injected, and serial images were acquired over the next 1.5 hours (15-sec. images for the first 2 min., then 1-min. images thereafter). Regions of interest (ROI) were drawn over the temporal, frontal, thalamic, parietal, and occipital areas through all slices and time-frames. Binding potentials (BP) were derived from these data for each ROI, using a reference tissue model with cerebellum as the reference tissue.
25 The cerebellum has no specific setoperone binding sites and is therefore appropriate as a reference tissue.
25,26Because age has been shown to affect serotonin metabolism,
27–29 the binding potentials (BPs) for each ROI were compared between groups, using analysis of covariance (ANCOVA), covarying for age.
Results
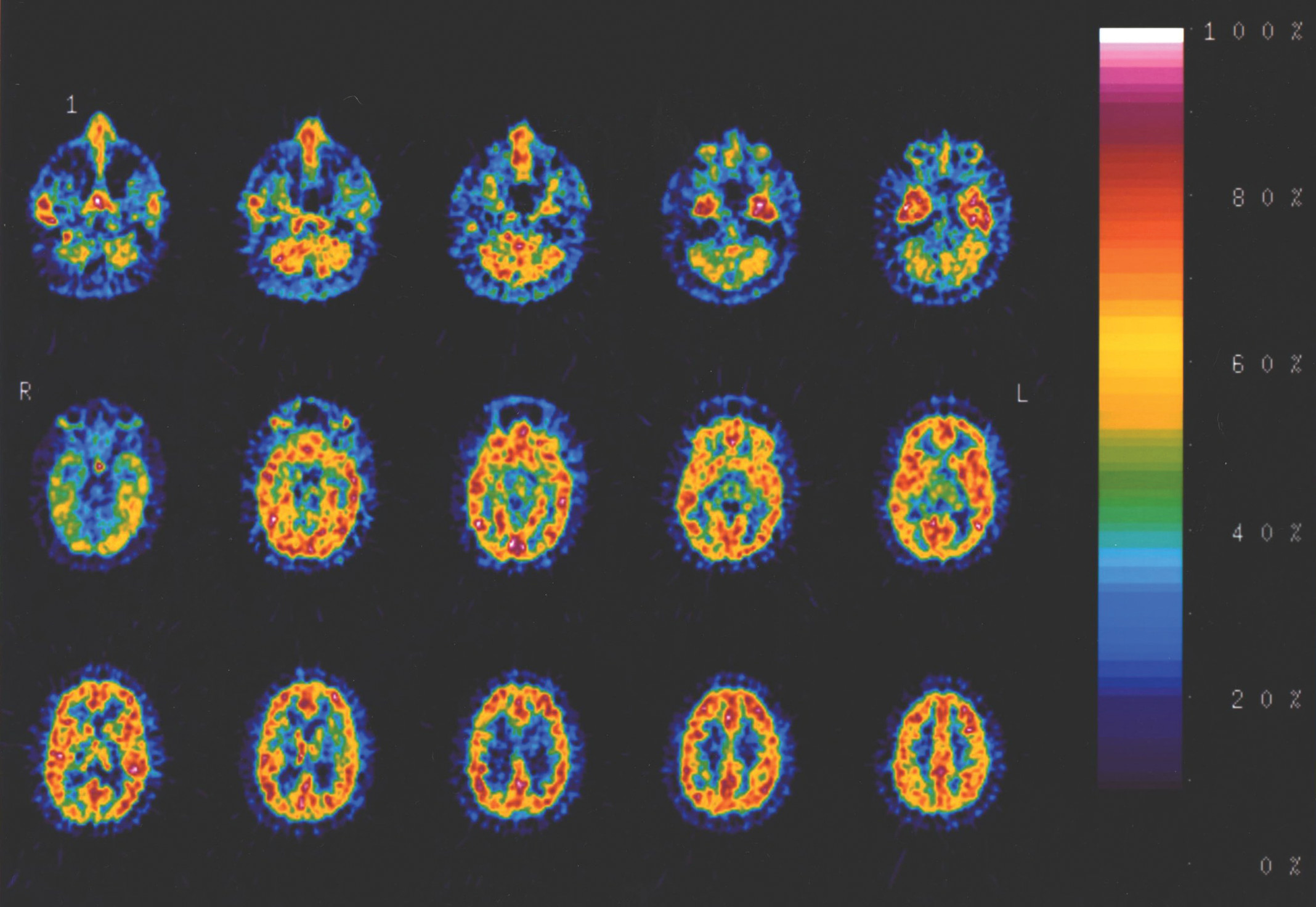
As described above, two subjects were excluded because of SSRI use. However, neither subject was an outlier for their group for BP. One remaining autism subject was an outlier because of infiltration of the dose during injection and was therefore excluded. One remaining control subject had the same BP (maximum BP for any ROI=0.41) as the subject with the infiltrated dose (maximum BP for any ROI=0.40) and was also excluded because of a suspected problem with the injection. Therefore, the total number of subjects included in the analysis was 6 individuals with autism and 10 control subjects. A typical image for one of the subjects with autism is shown in
Figure 1.
Comparison between groups, using ANCOVA, for each ROI and covaried for age, revealed that BP was lower for the thalamus in autism (
F[1,14]=4.82; p=0.047) than in controls. However, there was no significant decrease in BP for the other proposed brain regions: the frontal, temporal, parietal, or occipital regions (
Figure 2;
Table 1, top rows). Furthermore, the decrease in average BP for autism subjects did not reach significance for the comparison across all ROIs (
F[1,14]=2.13; NS). Also, as predicted (Bigham and Lidow, 1995;
27 Wang et al., 1995;
28 Pirker et al., 2000
29), a significant effect of age was observed (p=0.044) in the above ANCOVA, with BP decreasing with increasing age. The relationship between age and BP was the same for the autism group (Pearson correlation r = −0.816; p=0.048) and the control group (Pearson correlation r = −0.741; p=0.014), further demonstrating that the group difference did not result from any differential age effect between groups. Normal distributions were confirmed for all groups (p >0.1 for Shapiro-Wilk statistics for each ROI within each group), with no skewness detected (skewness <1.0 for all ROIs for both groups) among subjects included in the analysis. Furthermore, Levine’s test for equality of variances revealed equal variances across group for all ROIs.
To determine whether BP related to clinical aspects, we performed correlation analyses between regional BP and the Communication, Social Interaction, and Repetitive Behaviors scales on the ADI–R among the autism group subjects. BP in the thalamus significantly negatively correlated with ADI–R Communication scores (Pearson correlation r = −0.89; p<0.02), and a trend was shown toward a positive correlation with Repetitive Behaviors scores (Pearson correlation r=0.72; p <0.1). No other significant relationships were observed, and BP did not correlate with IQ scores for any ROI.
Because of the laterality effects observed by Chugani et al.,
11 we performed a secondary analysis, using ANCOVAs to examine laterality within subject and diagnosis between subjects, covarying for age, for each ROI, in order to detect side × diagnosis interactions. Whereas a number of trends were detected for decreased BP in autism for individual lateralized regions (
Table 1, bottom rows), a significant side × diagnosis interaction was detected for the thalamus (
F[1,14]=6.077; p=0.028), but not for the temporal, frontal, parietal, or occipital lobe. However, examination of the means revealed no significant difference between groups for either the right or left thalamus, covarying for age, and a greater difference between groups, although still not significantly different, was observed for the right thalamus than the left thalamus (
Table 1). This contrasts with the findings of changes in the left thalamus in autism reported by Chugani et al.
11Discussion
Serotonin binding to 5-HT
2 receptors appears to be decreased in the thalamus in the autism subjects. Significant differences were not detected in other brain regions with this population. A PET finding of decreased thalamic 5-HT
2 binding in autism
11 appears consistent with previous research revealing increased serotonin synthesis in autistic adults than in control subjects, which may result in down-regulation of the 5-HT
2 receptor. Another possibility is that increased synthesis occurs as a consequence of the inadequate activity of 5-HT
2 receptors in autism. This would need to be explored with further study if these thalamic findings are confirmed in future studies. Studies have revealed no clear genetic abnormalities in 5-HT
2 genes in autism,
30,31 but the presence of risk-factor genes affecting other aspects of the serotonergic system
32 tends to support the hypothesis of down-regulation of the 5-HT
2 receptors due to other factors. However, we did not detect the frontal lobe and lateralized thalamic effects in 5-HT
2, as reported for 5-HT synthesis,
11 although the older age of subjects in our study may also be a factor. These considerations must be considered preliminary, however, as these findings are pilot in nature.
The finding of a trend toward a relationship between BP and Repetitive Behaviors in this small sample of individuals with autism is not surprising, given the role of the serotonergic system in obsessive symptomatology.
19 However, the increased history of repetitive behaviors in this trend is unexpectedly associated with BP closer to the range of the control group. Given the small sample size, though, this would need to be confirmed in a larger sample. The more robust relationship between Communication and BP is in the expected direction, with lower BP (farther from the range of the control group) associated with a greater history of language impairment, which is of interest because of the role of the thalamus in language in relation to its cortical connectivity.
33 Further examination of these relationships is warranted in future studies.
The lack of significantly decreased overall 5-HT
2 binding, and 5-HT
2 binding in other regions of interest may be related to the small sample in this pilot study, particularly, given the previously described findings in ASD family members.
14 A larger study based on these pilot data is warranted, with monitoring of peripheral serotonergic markers, accounting for the potential impact of variations in regional anatomical volumes on apparent regional ligand binding, and with groups more closely matched for multiple demographics, or examining specific clinical subtypes of patients in order to account for and minimize heterogeneity. Assessment of family members as well as patients will be of interest, particularly, given the previously-mentioned findings on 5-HT
2 binding in ASD family members,
14 as well as the recent finding of decreased plasma serotonin in mothers of children with autism.
34 Also, forming a more complete picture of how serotonin metabolism differs in autism will require further examination of other aspects of the serotonergic pathway, either with other serotonergic PET ligands or with receptor binding of fast-frozen postmortem tissue. Initial studies of 5-HT
2 binding in fast-frozen tissue in autism have been negative in the hippocampus.
35 However, our finding of significantly decreased thalamic uptake in autism in this small sample remains of interest for future study.
Acknowledgments
Images were obtained at Massachusetts General Hospital, Boston, MA.
We thank Dr. Susan Manning, and our statistics consultant, Dr. Haikady N. Nagaraja, for their helpful comments on this manuscript.
This research was funded by a grant from the Stallone Fund, Los Angeles, CA.