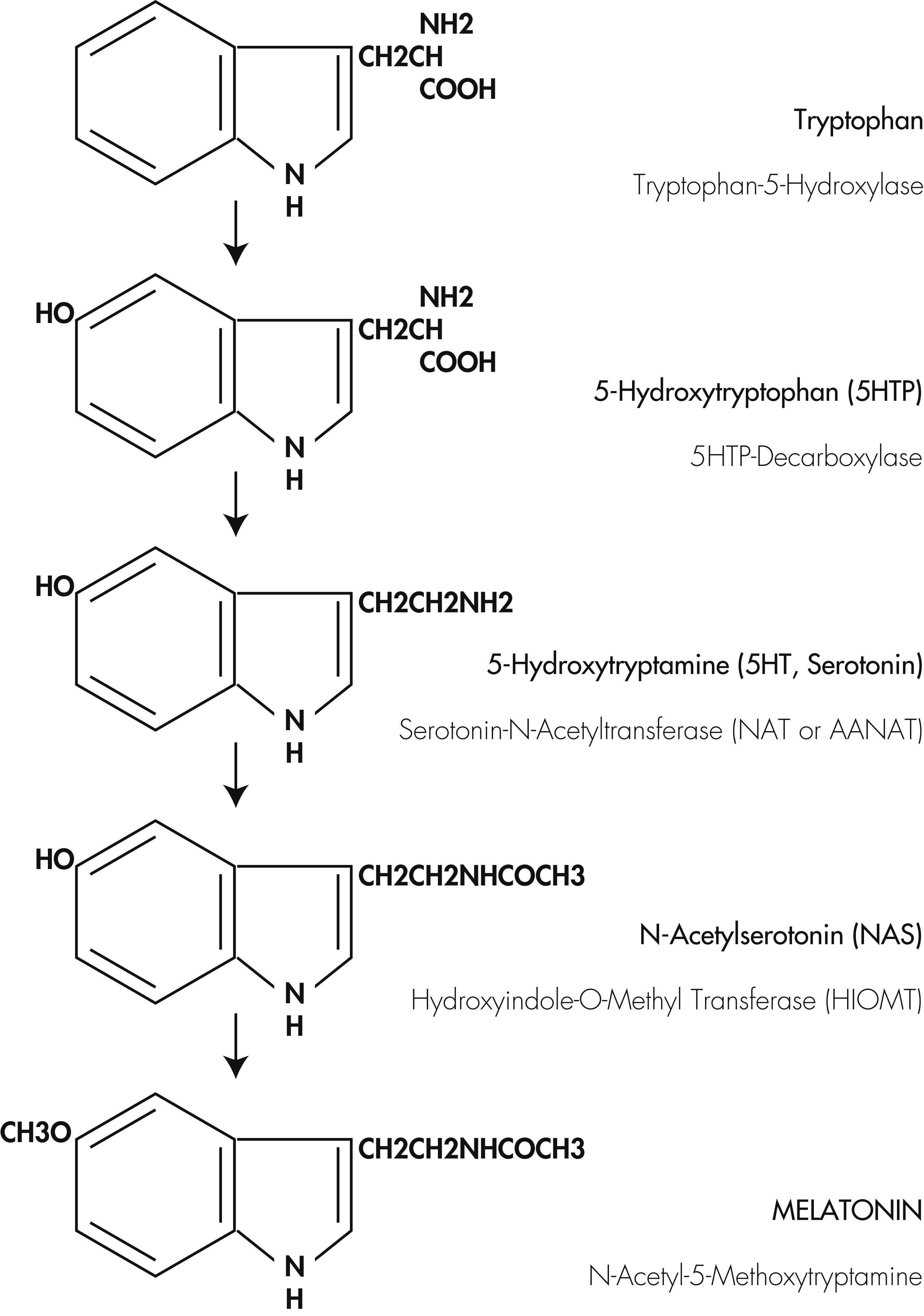
Depressive disorders constitute a heterogeneous group of disorders and involve complex interactions of genetic and environmental factors. Among the physiological factors that trigger this disease, disturbances of circadian and sleep–wake cycles, as well as abnormalities of melatonin secretion, have become the primary focus of attention
1 and formed the basis for the development of effective pharmacotherapeutic agents for treating this disease. Pharmacotherapy for treatment of depressive disorders have been in use since the 1950s, and includes tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), and selective serotonin reuptake inhibitors (SSRIs). All these antidepressants act through manipulations of monoaminergic neurotransmitter pathways in the brain and have been effective in causing remission of depressive symptoms in most of the clinical trials undertaken.
2–4 These drugs constitute the third most widely used class of antidepressants worldwide, with SSRIs alone accounting for 80% of the total market share.
5 With intensive epidemiological and EEG studies identifying “sleep and sleep–wake disturbances” as the most important underlying factor for the pathophysiology of depressive disorders,
6 the focus has shifted toward developing new classes of antidepressants that can correct the underlying abnormalities in sleep and circadian rhythms seen in patients with major depressive disorder (MDD) and bipolar disorders. Reports of significant correlations between low melatonin production and insomnia
7–9 suggest the possible relationship between melatonin and sleep. Use of slow-release melatonin in patients with depressive disorders improved sleep quality, but exerted only weak antidepressant effects.
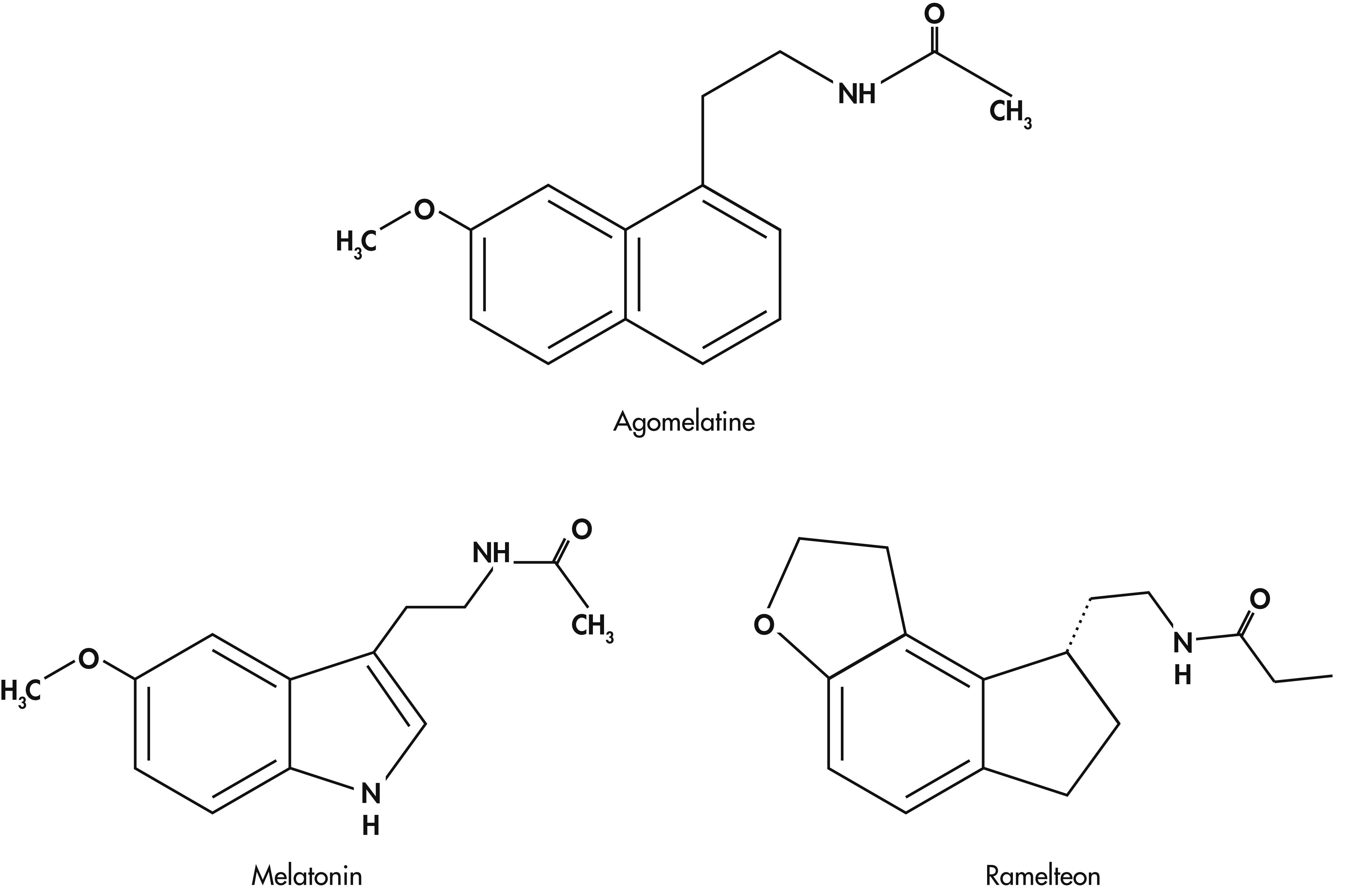
10 Development of a new synthetic analog of melatonin, namely agomelatine, a specific agonist of MT
1 and MT
2 melatonin receptors and a selective antagonist to 5-HT
2C receptors,
11 has been shown to have significant antidepressant properties.
12 Following these findings, the antidepressant efficacy of agomelatine has been demonstrated in a number of clinical studies undertaken in Europe, and it has been supported by earlier review studies.
13–17Melatonin, Depressive Disorders, and Sleep Disturbances
It is well known that patients with either major depressive disorder or bipolar disorders exhibit marked difficulties in the initiation and maintenance of sleep, poor quality of sleep, and frequent early-morning awakenings.
32–34 Also, the temporal distribution of REM sleep is typically altered during overnight sleep in depression, and this abnormality in the timing of the REM/non-REM cycle is attributed to the disorganized nature of the pathways that regulate the sleep–wake cycle.
34 The National Institute of Mental Health (NIMH) Epidemiologic Catchment Area (ECA) study of sleep disturbances and psychiatric disorders has identified sleep disturbance as a highly significant risk factor for subsequent development of depression.
35 Hence, persistent sleep abnormalities should be addressed first in treating depressive symptomatology.
36,37 A comprehensive program of therapy for depressive disorders should depend not only on clinical and behavioral symptoms, but also on the sleep and circadian rhythm disturbances of depressive disorders.
38,39 Accordingly, an ideal antidepressant should decrease sleep-onset latency, decrease the number of awakenings after sleep onset, and should increase alertness during daytime.
40 Currently, SSRIs constitute 80% of all prescription antidepressants,
5 but they have been found to exacerbate the sleep disturbances, and one-third of the patients receiving SSRIs also receive concomitant sedative-hypnotics.
41 Use of these sedative-hypnotics, consisting of benzodiazepine or nonbenzodiazepine drugs, can also result in many adverse effects, such as rebound insomnia, cognitive and memory impairment, dependency, and so on. Also, all the conventional antidepressants that are in use today (TCAs, MAOIs, SNRIs, SSRIs) elevate daytime mood by activating CNS mechanisms. If these energizing effects are sustained at night, they can very much reduce sleep efficiency and quality.
42 Hence, an ideal antidepressant, while elevating the mood during daytime, should also preserve the quality of sleep at night.
43 Agomelatine, a melatonergic agonist developed by Servier Laboratories, France, with a high affinity for MT
1 and MT
2 melatonergic receptors, and antagonism of 5-HT
2C receptors, has demonstrated its potential as an antidepressant in a number of preclinical studies and has also proved its clinical efficacy in patients with depressive disorders. This review will present the findings on agomelatine’s actions in animal models of depression as well as its clinical efficacy in patients with depressive disorders.
Agomelatine’s Antidepressant Effects: Preclinical Studies
Agomelatine has demonstrated its antidepressant activity in several animal models of depression, such as the forced swimming test,
49 psychosocial stress model,
50 learned-helplessness model,
12 transgenic mouse model,
46,51 and chronic mild stress model.
52 The antidepressant activity of agomelatine in various animal models of depression is summarized in
Table 1. The forced-swimming model has been used for assessing the antidepressant activity of a number of drugs. In this test, rodents are forced to swim in a situation where they cannot escape, as a result of which they become immobile, floating in an upright posture.
50 This is a validated test for antidepressant activity.
The acute administration of agomelatine either orally or intraperitoneally to rats or mice at 4 mg/kg, 16 mg/kg, or 32 mg/kg doses significantly decreased the duration of immobility in all the doses tested in rats.
49 But in mice, only repeated doses of agomelatine induced antidepressant-like effects in the forced-swimming model. The mechanism of antidepressant effect seen in this study was attributed to 5-HT
2C antagonism and to action on melatonin receptors.
49 The sucrose-consumption test after mild stress is used as one of the animal models of depression. By using this animal model, it was shown that administration of agomelatine at 10 mg/kg or 50 mg/kg doses counteracted the stress-induced decrease in sucrose consumption. Agomelatine was found to be more potent than melatonin in this antidepressant model. The role of MT
1 and MT
2 melatonergic receptors in mediating the antidepressant effect was evaluated by concomitant administration of the MT
1/MT
2 receptor-antagonist S22153, which inhibited the antidepressant effect of both agomelatine and melatonin and suggested the involvement of MT
1/MT
2 melatonergic receptors in mediating the antidepressant response.
51,52 In the learned-helplessness model test, the number of escape failures is evaluated to assess antidepressant efficacy. By using this model, the effects of agomelatine, imipramine, melatonin, and a selective 5-HT
2C antagonist were evaluated, and the effects of agomelatine were compared with other agents. Agomelatine (10 mg/kg BW) was given for 5 days once or twice daily, and the effects of pretreatment with S22153 (a melatonin-receptor antagonist; 20 mg/kg BW) were studied. A deficit in avoidance-learning was observed, but administration of agomelatine alone (10 mg/kg/BW) administered once a day significantly reduced this deficit. Because the effects of agomelatine were canceled by S22153 and not by SB-242084 (the 5-HT
2C receptor antagonist), it is suggested that melatonin receptors are involved in the mediation of agomelatine’s antidepressant effect.
53,54The transgenic mouse model with decreased glucocorticoid receptor (GR) expression is used for studying the antidepressant effects of drugs. Behavioral changes using the Porsolt forced-swim test and elevated plus-maze test were assessed in transgenic mice after administration of either agomelatine 10 mg/kg, melatonin 10 mg/kg, or desipramine 10 mg/kg. Drugs were injected intraperitoneally for the total period of 21 to 42 days, 2 hours before the onset of the dark period. Agomelatine reversed the decreased mobility in the forced-swimming test, and the same effect was noted with melatonin or desipramine. Even in the elevated plus-maze test, agomelatine reversed the behavioral changes.
51 The number of open-arm entries and total time spent were greatly reduced by agomelatine. In the same study, it was also noted that, after a phase-shift, agomelatine accelerated the phase-shift much more efficiently than melatonin, thereby showing its efficient resynchronizing effect, which indicates the therapeutic efficacy of agomelatine in treating depressive disorders.
51Depression is suggested to be due to desynchronization of various bodily rhythms, and correcting this underlying abnormality is thought to be critically important in correcting this disorder. The resynchronizing effect of agomelatine on disturbed circadian rhythms in experimental animals has also been studied earlier, and the effects of agomelatine on re-entrainment of disturbed circadian rhythms as studied by various investigators are presented in Table 1.
55–60 The effects of agomelatine in resynchronizing disturbed circadian rhythms are attributed to its actions on MT
1 and MT
2 melatonin receptors present in the SCN. This chronobiotic property of agomelatine is regarded as one of the main underlying factors in the antidepressant effects of agomelatine.
61Chronic social stress is one of the main triggering factors for the development of depressive disorders. On this basis, an animal model has been developed using tree shrews. Subordinate animals were subjected to psychosocial conflict daily by exposing them to dominant animals for 1 hour. The intensity of psychosocial stress in subordinate tree shrews was demonstrated by pronounced elevation of urinary cortisol levels, which reflects the sustained activation of the hypothalamic-pituitary-adrenal (HPA) axis. Chronically stressed tree shrews were injected with agomelatine 40 mg/kg for 28 days. Agomelatine treatment allowed subordinate animals to remain under psychosocial conflict situations without stress and normalized the activity of the HPA axis, as shown by the reduction of urinary cortisol levels.
62 By using impulse-related behavior, rats were trained in a T-maze and allowed to choose between two magnitudes of reward: immediate but small reward (getting two pellets) versus delayed but large reward (getting 10 pellets). The behavior of the rats was observed after administration of agomelatine (10 mg/kg and 30 mg/kg doses), melatonin 3 mg/kg and 10 mg/kg doses, clomipramine 8 mg/kg, fluvoxamine 4 mg/kg, and GR205171 (substance P receptor antagonist) 10 mg/kg and 30 mg/kg. Agomelatine, clomipramine, fluvoxamine, and GR205171 significantly increased the number of choices of the large-but-delayed reward. This delayed-gratification response chosen by those with agomelatine and other drugs reveals their ability to improve impulse-control, regarded as an antidepressant effect.
63 By using the chronic corticosterone animal model of depression and anxiety state (CCAMD), the behavioral consequences of either chronic agomelatine (10 mg/kg–40 mg/kg per day) or fluoxetine (18 mg/kg per day) were assessed in a number of paradigms such as the forced-swimming test, open-field paradigm, novelty-suppressed feeding (NSF), and the splash test (ST). Also, the effects of agomelatine on neurogenesis in the ventral and dorsal hippocampal regions were analyzed. Both agomelatine and fluoxetine were administered for a period of 4 weeks. Results of this study from the forced-swimming test, a well-recognized screening test for depression, shows that agomelatine at both doses (10 mg/kg and 40 mg/kg per day) and fluoxetine increased mobility duration in corticosterone- and noncorticosterone-treated rats. All multiple behavioral parameters with agomelatine and fluoxetine were found effective in reversing depression/anxiety-like phenotypes induced by excess glucocorticoids.
64 The effect of agomelatine also was assessed on dorsal and ventral hippocampal regions. The ventral hippocampal region is implicated in anxiety and mood regulation,
65,66 whereas the dorsal hippocampus is concerned with spatial memory. Assessment of the effects of agomelatine or fluoxetine on neurogenesis of dorsal and ventral hippocampal regions revealed that cell proliferations in corticosterone-treated rats were similar in both dorsal and ventral hippocampal regions.
64 Agomelatine was able to reverse the decreased cell-proliferation induced by corticosterone in the whole hippocampal region. Besides these effects, agomelatine increased the light/dark ratio and reversed the alterations in this ratio induced by corticosterone treatment, suggesting the normalization of disturbed circadian rhythms. Thus, all the parameters assessed in this study including antidepressant effect, normalization of disturbed circadian rhythms, and neurogenesis of hippocampal regions strongly suggest agomelatine as a new, innovative, antidepressant drug.
67Agomelatine’s Therapeutic Efficacy in Depressive and Anxiety Disorders
The first report on the clinical efficacy of agomelatine in the treatment of MDD was undertaken by Loo and his associates (2002).
14 The report is based on the clinical trials undertaken in multi-national, multi-center, double-blind, placebo-controlled investigations involving 711 patients drawn from 102 clinical centers in Belgium, the U.K., and France. Of these 711 patients, 67.1% met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for recurrent MDD, with 33.5% of patients having an episode of severe intensity. The mean baseline score on the 17-item Hamilton Rating Scale for Depression (Ham-D) was 27.4. Either agomelatine (25 mg/day) or paroxetine (20 mg/day) was administered for a total period of 8 weeks. By using remission analysis, the authors found that both agomelatine (30.4%) and paroxetine (25.7%) brought about significant remission when compared with placebo. Responder analysis (defined as 50%-or-more reduction in the baseline score of the Ham-D) showed agomelatine to be superior (61.5%) to placebo (46.3%), whereas paroxetine did not differ much (56.3%) from the placebo response. Among the 711 patients, a subpopulation of patients was categorized as severely depressed (586 patients with Ham-D score >25). Administration of agomelatine in this subpopulation produced a significant response, as compared with placebo (p<0.05), whereas the paroxetine response did not differ much from the placebo effect. In addition to its effects on depressive symptoms, both agomelatine and paroxetine reduced anxiety symptoms. Similar to the findings reported from the above study, a second multi-center and multi-national study involving 21 centers across Finland, Canada, and South Africa, involving 212 patients (age 18–65 years) evaluated the clinical efficacy of agomelatine.
68 In this study, the Ham-D score exceeded 22. This double-blind, placebo-controlled trial of agomelatine (25 mg to 50 mg) was carried out for 6 weeks. In this study, the intent-to-treat (ITT) group consisted of 106 patients. Treatment with agomelatine for 6 weeks was associated with significant improvement in the clinical status of the patients (p=0.045) as compared with the placebo response. Agomelatine (25 mg to 50 mg) reduced the Ham-D score quite significantly in the severely depressed subgroup with Ham-D scores exceeding 25. In this group, the agomelatine response was more effective than placebo (p=0.024). The significantly higher rate of responders to agomelatine (49.1%) versus placebo (34.3%) and the shorter time to first clinical response further lend support to the view that agomelatine is effective especially in patients with greater symptom severity.
The availability of antidepressants with efficacy in severely depressed patients is very important from the clinical point of view because this group is relatively resistant to current antidepressant therapy, which relies heavily on SNRIs or SSRIs.
69 Hence, it is suggested that the greater clinical response seen with agomelatine when compared with that of other antidepressants (SSRIs) points to the superior efficacy of agomelatine over these other antidepressants. In another study, carried out for 12 weeks in 277 subjects, the patients were randomized to receive agomelatine at a dose of 50 mg/day or venlafaxine XR (extended release) at two different doses, namely, 75 mg/day for the first 2 weeks and then increased to 150 mg. The rates of remission were found to be 73% for agomelatine and 67% for venlafaxine-XR-treated patients. In a flexible-dosing, 6-week trial in 167 patients, subjects were assigned to receive either agomelatine (25 mg–50 mg) or venlafaxine (75 mg–150 mg) in the immediate-release form. A significant reduction in Ham-D scores was found in both groups, with agomelatine reducing the Ham-D score from 25.9 (SD: 3.2) to 9.0 (SD: 5.4), and venlafaxine reducing the Ham-D score from 26.0 (SD: 3.3) to 8.9 (SD: 5.2); the response rates were, respectively, 76% and 71% for agomelatine and venlafaxine immediate-release.
15,70 To evaluate the sleep efficacy of agomelatine, a placebo-controlled, double-blind study was undertaken in 332 patients with MDD for a period of 6 weeks. Agomelatine was administered at a dose of 25 mg–50 mg/day, and venlafaxine was given at a dose of 75 mg–150 mg/day. Although sleep quality, as measured by the Leeds Sleep Evaluation Questionnaire (LSEQ), was found better with agomelatine, the antidepressant effect was found similar for both drugs.
67Agomelatine’s superiority in treating patients with MDD was studied in another 6-week, double-blind, parallel-group study, involving 238 patients.
13 Agomelatine was administered at 25 mg/day to these patients, and this dose was raised to 50 mg/day after 2 weeks in patients who showed negligible improvement. Agomelatine was found to be significantly (p<0.001) superior to placebo, with a difference of 3.44. The response rate was 54.3% (agomelatine) to 35.5% (placebo). Agomelatine improved depressed mood and sleep items of the Ham-D score quite considerably. The drug was well tolerated and found to be safe in these patients.
13Evaluation of the efficacy of agomelatine on depressive symptoms in patients with major depressive disorder was carried out in an open-label study of 30 MDD patients receiving flexible doses of 25 mg–50 mg/day. Of these, only 24 patients (80%) completed 8 weeks of treatment. Agomelatine treatment produced an early response, and significant improvement was noted in all these patients, as seen in Ham-D scores. Moreover, the effect of agomelatine in improving anhedonia was noted for the first time in this study.
71The efficacy of agomelatine in preventing the relapse of depressive symptoms and improving the clinical status of patients with MDD was assessed in a 32-week study on 165 patients. In this study, patients with DSM-IV major depressive disorder who responded to 8- to 10-week administration of agomelatine (25 mg–50 mg/day) were randomly assigned to receive continuation of treatment with agomelatine (N=165) or placebo (N=174) for the treatment period of 24 weeks. The main outcome was time-to-relapse. During this 6-month evaluation period, the incidence of relapse was found to be significantly lower in patients who continued their treatment with agomelatine than those who switched over to placebo (p=0.0001). The cumulative relapse rate with agomelatine was 21.7%; and for placebo, it was 46.6%. The findings of this study support the concept that agomelatine is an effective and safe antidepressant for continuation therapy. This long-term study confirms the earlier reports of agomelatine’s efficacy for short-term therapy.
13Agomelatine 25 mg/day was also used as an adjunctive treatment along with either lithium (N=14) or valpromide (N=7) in an open-label study of bipolar I patients. Agomelatine was administered for a minimum period of 6 weeks, followed by optimal extension up to an additional 46 weeks. Using intent-to-treat data, it was found that 81% of the patients met the criteria for marked improvement. Patients belonging to the severe category of depression, with Ham-D score over 25.2 (47.6% of the total number of patients), responded as early as the first week of treatment. Nineteen patients entered the optional extension for a mean of 211 days (6–325 days), and, of these, 11 patients completed a 1-year extension of treatment. Agomelatine was found to be an effective antidepressant in this study.
72 (See
Table 2 for a listing of studies on depression treatment.)
Besides being effective in treating MDD and bipolar I disorder, agomelatine also has been tried for depressed patients with seasonal affective disorder (SAD). In this open-label study the efficacy of agomelatine (25 mg/day) was evaluated for a period of 14 weeks. Assessment of agomelatine’s efficacy was evaluated by using various psychometric scales, including the Structured Interview Guide for the Hamilton Depression Rating Scale (SAD version; SIGH–SAD); the Clinical Global Impression of Severity (CGI–S); the Clinical Global Impression of Improvement (CGI–I); the Circascreen, a self-rating scale for the assessment of sleep and circadian-rhythm disorders; and the Hypomania Scale. Agomelatine use in these patients caused a progressive and statistically significant decrease in SIGH–SAD, CGI–S, and CGI–I scores beginning in the second week of treatment. Also, the scores on the Circascreen improved quite substantially after agomelatine (p<0.001). Treatment with agomelatine for 14 weeks yielded a response rate of 75.7% (defined as a SIGH–SAD score <50% of the baseline value) and a remission rate (SIGH–SAD <8) of 70.3% in the intent-to-treat sample. The efficacy of agomelatine in treating patients with seasonal affective disorder was demonstrated in this study. The drug was well tolerated throughout the study, and there was only one report of an adverse effect, mild fatigue, showing thereby that the overall rating of agomelatine is good.
73Agomelatine has been found effective not only in animal models of depression but also in animal models of anxiety.
16,74 The clinical efficacy of agomelatine has also been studied in 121 DSM-IV GAD patients randomized to agomelatine (25 mg–50 mg/day) or placebo for 12 weeks. Analysis of covariance of change in the last Hamilton Rating Scale for Anxiety (Ham–A) score from the baseline score demonstrated significant superiority of agomelatine in a 25 mg–50 mg/day dose, as compared with placebo. From this finding, it was concluded that agomelatine is an effective therapeutic drug for the treatment of generalized anxiety disorder.
75Agomelatine’s Antidepressant Effect: Mechanism of Action
It is a longstanding dictum that all available antidepressants exert their therapeutic actions mainly by the modulation of monoaminergic mechanisms in the brain. Depressive patients often experience a number of sleep disturbances, like difficulty in falling asleep, staying asleep, disturbed nocturnal sleep, early-morning awakening, etc.
36 A number of studies point out that depression is linked to disturbances in circadian rhythms; hence, an antidepressant that benefits sleep quality and resets disturbed circadian rhythms will have more beneficial therapeutic antidepressant efficacy. As noted in the earlier sections, agomelatine is a melatonergic agonist of MT
1/MT
2 melatonergic receptors, with antagonism of 5-HT
2C serotonergic receptors. The therapeutic efficacy of agomelatine in depressive disorders is attributed to its action on MT
1 and MT
2 melatonergic receptors, present largely in the SCN of the hypothalamus, and also to its 5-HT
2C antagonism.
54,81 5-HT
2C receptors are concentrated in the frontal cortex, amygdala, hippocampus, cortico-limbic structures, and SCN, and these structures are involved in the regulation of mood and cognition.
82 Antidepressants in use have been shown to exert their therapeutic effects by decreasing the number of 5-HT
2C receptors;
83 but agomelatine’s superiority over other antidepressants with 5-HT
2C antagonism has been related to its effects in “improving sleep and daytime alertness.”
84,85 As we have seen earlier, agomelatine exerted a superior antidepressant effect in animal models of depression, whereas neither melatonin nor 5-HT
2C antagonist antidepressants could mimic the antidepressant effect of agomelatine.
54 Activation of both melatonergic MT
1 and MT
2 receptors and blockade of 5-HT
2C receptors are essential for agomelatine’s antidepressant effect.
17 In patients with MDD, agomelatine has been shown to improve all aspects of the sleep–wake cycle as early as during the first week of treatment itself.
86 This action of agomelatine in improving sleep efficiency and normalizing disturbed sleep–wake cycles is an important mechanism by which agomelatine exerts its therapeutic antidepressant effect.
39 Current antidepressants that are in clinical use today, especially SSRIs, cause profound sleep disturbances and exacerbate insomnia. Hence, sleep medications are used as a combination therapy along with antidepressants when treating patients with depressive disorders. As has been discussed earlier, all antidepressants elevate daytime mood in depressed patients by activating CNS arousal mechanisms, but since this effect is sustained throughout the 24-hour period, they can cause disruption of sleep mechanisms.
42 The effectiveness of the novel antidepressant agomelatine is considered to be due to its dual actions of preserving sleep quality and efficiency through melatonergic MT
1 and MT
2 activation and elevating mood and activity through serotoninergic 5HT
2C antagonism. Agomelatine antagonizes the 5-HT
2C receptors both during daytime and at night.
Circadian Pacemaker in the Suprachiasmatic Nuclei (SCN)
All living organisms exhibit robust physiological and biochemical rhythms.
87 These rhythms depend upon the presence of clock genes in the cells and are synchronized by a master clock located in the suprachiasmatic nuclei (SCN) of the hypothalamus.
88 Circadian periodicity generated by the SCN is approximately 24.2 hours
89 and is synchronized to exactly 24 hours by the environmental light–dark cycle that acts through the retina and the retino–hypothalamic tract.
90 Neurons in the SCN, as has been earlier noted, contain both MT
1 and MT
2 melatonin receptors. The circadian rhythm of melatonin secretion is regulated by the SCN, and melatonin is also a feedback regulator of the SCN by acting through both MT
1 and MT
2 melatonergic receptors. Both phase and amplitude of circadian rhythms are influenced by melatonin, acting through these receptors. Phase-shifting of circadian rhythms by melatonin are effected through MT
2 melatonergic receptors,
91,92 whereas the amplitude of circadian rhythms as studied by neuronal firing rates in SCN are influenced by melatonin acting through MT
1 melatonergic receptors.
93,94 As depressive disorders have been suggested to be due to disorders of circadian rhythms, including sleep–wake rhythms, it is likely that the actions of agomelatine in resetting the disturbed rhythms and sleep–wake rhythms is mediated through melatonergic receptors of the SCN.
The role of melatonin receptors in mediating antidepressant effects has been inferred from studies carried out on MT
1 melatonin-receptor knockout mice (MT
1−/−). MT
1 melatonin-receptor knockout mice exhibit depression-like behavior. Both male and female melatonin-receptor knockout mice spent significantly more time in immobility in the forced-swimming test, a test that is usually employed for studying animal models of depression.
86 Given that the disruptions in the circadian rhythms and sleep–wake cycles correlate with the severity of depression,
85 the chronotherapeutic effect of agomelatine in MDD was evaluated. Agomelatine caused an increase in relative amplitude of the circadian rest–activity cycle by the end of Week 1, and it ran parallel with improvements of sleep efficiency and sleep latency from Week 1 to Week 6. Depression and anxiety symptoms were very much improved in these patients, along with circadian rhythm and sleep improvements.
75 This study supports the concept that agomelatine’s specific target of action is mainly on MT
1/MT
2 melatonergic receptors in the SCN, and, thereby, it corrects the underlying abnormality of the disturbed circadian rhythm and sleep–wake cycles of patients with depressive disorders. Hence, the important component of agomelatine’s antidepressant effect resides in the mechanism of improving sleep efficiency coupled with the correction of disrupted circadian rhythms.
95 Recent review studies have presented evidence supporting the clinical supremacy of agomelatine as an effective antidepressant,
96 because of its early onset of action,
97 low relapse rate,
98 and targeting of melatonergic receptors for normalizing disturbed sleep and circadian rhythm.
99–102Neurogenic Effects of Agomelatine: Another Possible Mechanism for Its Antidepressant Effect
Recent preclinical studies have demonstrated that agomelatine, like other antidepressants, such as SSRIs and tricyclics, increase cell proliferation in the dentate gyrus of adult rats
103,104 Chronic agomelatine treatment reversed the decreased neurogenesis of glucocorticoid receptor-impaired mice (GR-mice), an animal model of depression,
105 and this effect was shown to occur mainly in the ventral hippocampus.
103,104 The ventral hippocampus is implicated in anxiety and mood-regulation, and the dorsal hippocampus is concerned with spatial memory.
65,66,106 Using corticosterone-treated mice (an animal model of depression and anxiety), the effects of agomelatine or fluoxetine were tested on dendritic maturation in both dorsal and ventral hippocampal regions. Although both antidepressants modified the maturation index, the number of double cortin expression cells (DCX+ cells) with tertiary dendrites was increased with agomelatine (10 mg/kg–40 mg/kg day) only in the ventral hippocampal region of corticosterone-treated animals.
64 Agomelatine induced an early acceleration of cell maturation at 8 days of development.
104 Previous studies point out that the earliest time-point at which an antidepressant (fluoxetine) caused cell maturation was 21 days.
107 From this study, it is evident that agomelatine has a more rapid action on cell maturation than SSRIs or any other monoaminergic antidepressants.
104 Because agomelatine caused dendritic maturation in animal models of depression/anxiety, with earlier onset of action, and also demonstrated circadian-rhythm regulatory effects in animal models of depression, it contributes a distinctive profile in its antidepressant action.
64 As the ventral hippocampus projects to the prefrontal cortex and amygdala, these agomelatine data support the view that the ventral hippocampus is involved in the emotional circuitry supporting the control of depressive/anxiety states.
65,108 The ventral hippocampus seems to contain 5-HT
2C receptors, but actions of agomelatine on dendritic maturation are thought to be mediated through both melatonergic and 5-HT
2C receptors present in the ventral hippocampus.
104 Hence, based on agomelatine’s action in improving sleep efficiency, resynchronizing disrupted circadian rhythms, and enhancing dendritic maturation, demonstrated in both preclinical and clinical studies, it is clear that agomelatine has a novel antidepressant effect, with a rapid onset of action and greater clinical efficacy.
The effects of 25 mg/day–50 mg/day of agomelatine (N=154) or sertraline (50 mg/day–100 mg/day) was studied in patients with MDD over a period of 6 weeks in a randomized, double-blind study. With agomelatine, significant improvement in the relative amplitude of the rest–activity cycle was observed in the first week (p=0.01); improvements in sleep quality (p<0.001) and sleep efficiency (p<0.001) were also observed from Week 1 to Week 6. Depressive symptoms improved considerably more with agomelatine (p<0.05) than with sertraline. This study supports the idea that agomelatine is more efficient in improving sleep parameters, the circadian rest–activity cycle, and depressive symptoms than sertraline.
109Additional Heuristic Melatonergic Antidepressant and Anxiolytic Mechanisms
The effects of improved sleep and MT1/MT2 stimulation can also exert important effects on depressive and anxiogenic mechanisms that include oxidative stress, nitric oxide metabolism, mitochondrial function, neuroinflammation, neurotrophins (e.g., BDNF and GDNF), dopaminergic integrity, cyclic nucleotides (cAMP and cGMP), clock gene expression (Clock and NPAS2), heat shock proteins (HSP27), and apoptosis.
As mentioned above, sleep-enhancing effects may be important to agomelatine’s therapeutic effects. More specifically, improvement of sleep may target certain mechanisms implicated in depressive and anxiety pathophysiology. Impaired sleep has been shown to adversely affect oxidative stress,
110 mitochondrial integrity and function,
111 and inflammation,
112 mechanisms that play pathophysiological roles in mood and anxiety disorders. Specifically, oxidative stress has been related to major depression,
113–117 especially with regard to the hippocampus.
118 Oxidative stress has also been implicated in bipolar disorder
119–125 and anxiety.
126–128 Mitochondrial perturbations have been linked to major depression
114,129–131 and bipolar disorder.
129,130,132 Inflammation has also been associated with major depression
116,131,133–135 and bipolar disorder.
132,136–138 For example, sleep deprivation is thought to trigger an inflammatory and stress response in the brain through gene induction.
112 Improvement of sleep by agomelatine can therefore improve oxidative, mitochondrial, and inflammatory processes that contribute to the pathophysiology of these disorders.
Besides the SCN and several other hypothalamic regions, melatonin receptors can also be found in the paraventricular nucleus of the thalamus, parabrachial nuclei, and olfactory bulb in mice.
139 In lizards, they are observed in visual pathway centers, the striatum, habenula, mammillary nucleus, septum, interpeduncular nucleus, medial cortex, and dorsal cortex.
140 In rabbits, MT receptors were found in the cerebral cortex, cingulate gyrus, and hippocampus.
141 MT
1 receptors are found in human cerebellar neurons.
142 MT
2 receptors are distributed in the human hippocampus
143 and cerebellar glia.
142 Limbic structures, striatum, cerebellum, hippocampus, and cingulate gyrus have each been demonstrated to play important roles in human mood disorders. Effects on MT
1 and MT
2 receptors in these areas might therefore mediate the antidepressant and anxiolytic effects of agomelatine.
Direct agonist effects of agomelatine on melatonin MT
1 and MT
2 receptors may also remedy relevant pathophysiological processes by protecting against oxidative stress, including effects on nitric oxide (NO) levels. Protection against oxidative stress appears to be mediated through MT
1144–147 and possibly, MT
2146,147 receptors. NO is important in mediating major depression
148 and bipolar depression
149 pathophysiologies. In bipolar depression, serum NO is elevated, and normalizes with antidepressant treatment over 30 days.
149 In rat intestinal synaptosomes, melatonin reduced NO synthase activity, an effect possibly mediated by the MT
1 receptor.
150 MT
1 and MT
2 receptor stimulation also independently increases brain-derived neurotrophic factor (BDNF). BDNF is involved in the pathophysiologies of both major depression
151–153 and bipolar depression.
154,155 Although stimulation of either MT
1 or MT
2 receptors increase BDNF concentrations,
156 agomelatine additionally increases BDNF through a synergistic effect of MT
1, MT
2, and 5HT
2c receptors.
104,157As noted earlier, MT
1 stimulation inhibits adenylyl cyclase cAMP production,
158 consistent with cAMP down-regulation that has similarly been correlated with the antidepressant effects of salbutamol
159 and imipramine.
160 MT
1 receptor activity in particular has been correlated with neurotrophic increases in BDNF,
156,161 glial-derived neurotrophic factor (GDNF),
161 and tyrosine hydroxylase.
162 There is evidence that GDNF levels are reduced in depressive disorders
163,164 and that increased GDNF-release constitutes an antidepressant mechanism.
165 GDNF further promotes dopaminergic neuron survival;
166 these neurons are involved in mediating depressive symptomatology.
167–169 A reduction of striatal dopamine-transporter binding is observed in seasonal affective disorder,
170 and dopamine seems to play a role in the semiology of this disorder.
171 These findings of improved dopaminergic neuron survival
166 and increases in tyrosine hydroxylase seen with MT stimulation
162 suggest protective and trophic effects on mood-related dopamine neurons. MT
1 effects on clock genes have also been suggested to participate in the pathobiology of mood- and dopamine-related behaviors.
172 MT
1 stimulation down-regulates Clock and up-regulates neuronal PAS-domain protein 2 (NPAS2) mRNA expression in mouse striatum.
172 Subjects with depression exhibit increased leukocyte clock mRNA expression.
173 Several Clock
174–176 and NPAS2
174,177,178 single-nucleotide polymorphisms have been linked to MDD
174 and seasonal affective disorder,
177,178 as well as to bipolar depression
174,177,178 symptoms
175 and recurrence.
176MT
2 receptor activation inhibits NO-induced increases in cyclic GMP
179—of interest because plasma cGMP levels have been noted to be increased in patients developing depression after receiving 6 weeks of interferon treatment.
133 MT
2 receptor-stimulation increases BDNF concentrations,
156 induces heat-shock protein HSP27, and prevents apoptosis,
180 perhaps related to agomelatine’s antidepressant effect,
115 since apoptotic markers are increased in depressive disorders
113,181,182 and are normalized by antidepressant treatment.
182Thus, multiple effects of agomelatine over a wide range of pathophysiological processes may bring about the observed benefits in major depression, bipolar disorder, seasonal affective disorder, and generalized anxiety disorder.
A last observation regarding the mechanism(s) of action of agomelatine should be kept in mind. Agomelatine displays a similar dissociation constant (K
d) for MT
1/MT
2 melatonin receptors to that of melatonin itself; that is, 10 nM at 10 p.m.
102 This fact implies that doses of 1 mg–3 mg melatonin or agomelatine are enough to saturate both MT
1/MT
2 receptors in the body. However, the therapeutic doses of agomelatine normally range between 25 mg/day and 50 mg/day, suggesting that an effect other than binding to MT
1/MT
2 receptors (and 5-HT
2c receptors) might take place. In this regard, it was recently reported that melatonin does not move freely through the body, as has earlier been suggested. Instead, subcellular melatonin distribution is a regulated process, even in the brain;
183 so studying the intracellular distribution of agomelatine may yield important information regarding intracellular effects of this melatonin analog.