Posttraumatic stress disorder (PTSD) is a psychiatric illness that affects individuals exposed to a life-threatening event or trauma.
1 The lifetime prevalence of PTSD is approximately 6.8% in the general United States population,
2 but has been estimated to be 19% in Vietnam veterans, with 9% suffering from PTSD symptoms more than 10 years post-war experience.
3 Similarly, PTSD rates in soldiers returning from the Iraq and Afghanistan conflicts have been estimated at 22%.
4 PTSD is associated with a great deal of suffering from psychiatric and physical comorbidities,
5 and it is likely to become an extremely pressing public health concern as more soldiers return from continuing operations.
In addition to the three core PTSD symptom clusters (intrusive recollections, avoidant/numbing symptoms, and hyper-arousal symptoms
1), investigators have shown that 1) PTSD results in neurocognitive deficits;
6–10 and 2) PTSD symptom severity is positively associated with degree of cognitive impairment.
11 Also, a meta-analysis revealed that verbal memory deficits are the most consistent cognitive impairment in PTSD patients,
12 just as memory impairment is the first notable symptom in Alzheimer disease (AD) patients.
13These observations led us to examine the prevalence of dementia in veterans with chronic combat-related PTSD (CR-PTSD). In that study,
14 we examined a large veteran cohort of patients with PTSD but no Purple Heart (PTSD+/PH−, N=3,660); those without PTSD but with a Purple Heart (PTSD−/PH+, N=1,503); those with PTSD and a Purple Heart (PTSD+/PH+, N=153); and those without PTSD or a Purple Heart (PTSD−/PH−, N=5,165). The incidence of dementia during the 9-year follow-up period was 2.2-fold higher (p<0.001) in the PTSD+/PH− group than the PTSD−/PH− group and 1.7-fold higher (p<0.001) than the PTSD−/PH+ group even after accounting for age, sex, race, number of primary care visits, and multiple comorbid illnesses (diabetes mellitus, dyslipidemia, hypertension, coronary artery disease, stroke, traumatic brain injury, alcohol abuse and dependence, and drug abuse and dependence). Notably, a second study also found a similar twofold increased risk of dementia in PTSD veterans as compared with veterans without PTSD.
15 The reasons for this association were unclear. We wondered whether neuroanatomical changes associated with PTSD might put these veterans at greater risk for dementia.
Methods
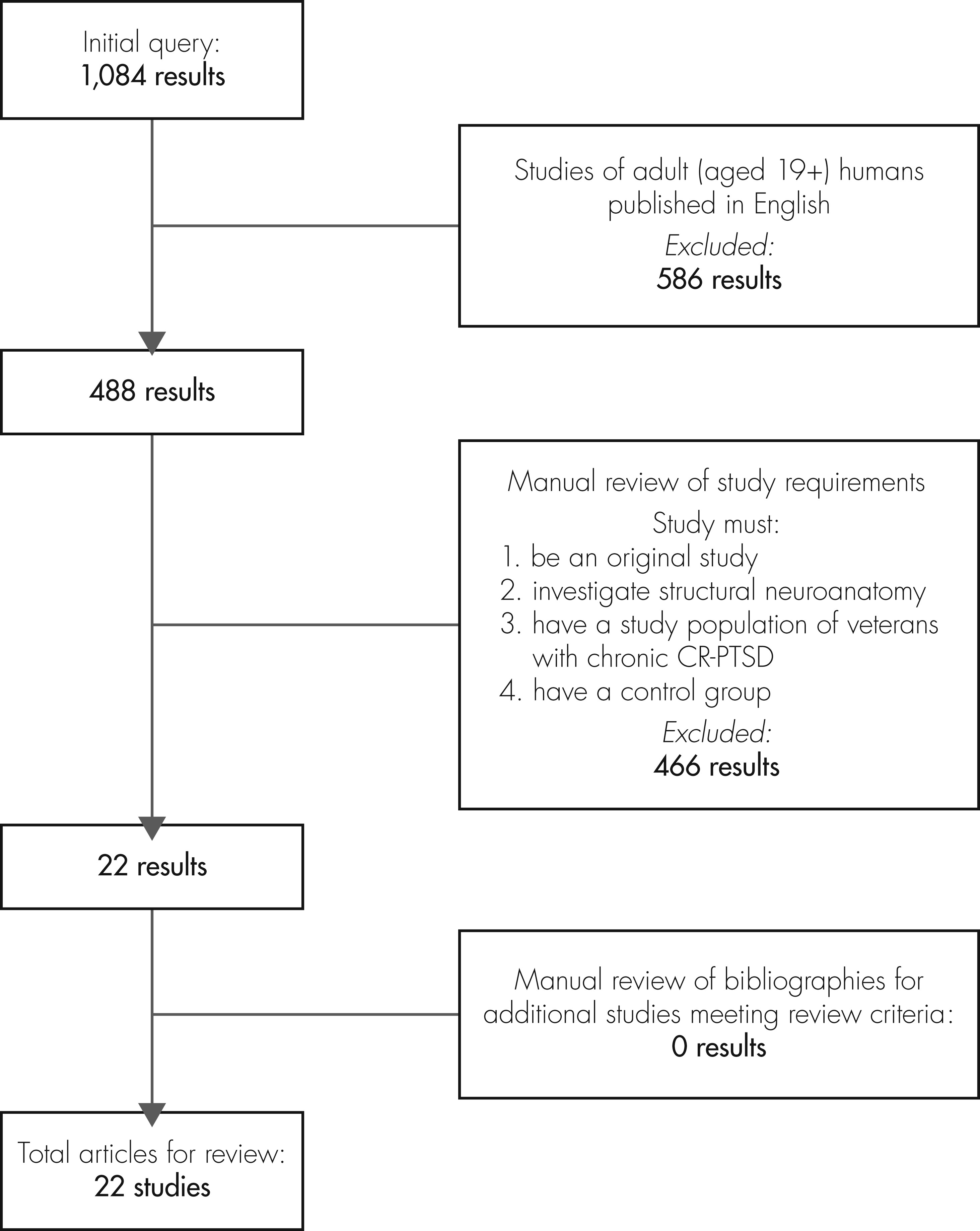
We used the PubMed database to search for the term PTSD in combination with any of the following terms: physical changes, neuroanatomical, frontal, parietal, temporal, hippocampal, cortical, prefrontal, amygdala, and locus coeruleus. The literature search extended to 08/11/2011 (range: earliest returned article, 1966 – latest returned article, 2011), and the articles produced for each of the above search combinations were merged to form a catalog of 1,084 articles. This initial query was filtered by including only human adult (age 19+ years) studies published in English (488 articles).
The resulting collection of articles was then reviewed for focus, demographics, and duration of PTSD by researcher personnel (JC). Each study had to 1) be an original study; 2) investigate structural neuroanatomy; 3) use veterans with chronic CR-PTSD, defined as PTSD of ≥6 months’ duration resulting from trauma in combat; and 4) compare the veteran group with a control group. This process produced 22 articles
18–39 that covered 21 cross-sectional studies
18–38 and one longitudinal study.
39 The bibliographies of these 22 articles were searched to identify relevant studies not captured by our search net, but none were identified (
Figure 1).
Review Process
Two authors (EM, JC) independently rated the quality of the selected 22 articles, using a scale developed for this study. The scale assigned each paper a score between 0 and 4, giving 1 point each for 1) having 10+ participants in the CR-PTSD group, based on guidelines for metaanalyses on imaging literature;
40 2) using a valid PTSD diagnostic tool (e.g., the Clinician-Administered PTSD Scale [CAPS], Mississippi Scale for Combat-Related PTSD); 3) using a combat-exposed control group without PTSD; and 4) accounting for substance abuse, given its prevalence in PTSD and association with brain atrophy.
41 In our opinion, higher-quality scores represent a stronger methodology for the purposes of this review.
Results were generated by abstracting all data related to structural neuroanatomy associated with PTSD. Specifically, we examined all statistical analyses that compared neuroanatomical volumes between chronic CR-PTSD veterans and a control group. For a finding to be considered positive, the reporting study had to demonstrate a significance level of ≤0.05.
Discussion
In the 22 studies reviewed, the most frequently cited neuroanatomical differences found in patients with chronic CR-PTSD were in the hippocampus, involving either smaller total or right hippocampal volumes. Although volumetric differences were reported in other regions, including the frontal cortex, temporal cortex, and ACC, the findings for these areas were less conclusive and preclude a firm conclusion.
The reductions in hippocampal volume observed in these studies offer a potential explanation for the increased rates of dementia we and others observe in veterans with chronic CR-PTSD.
14,15 Dementia is a loss of cognitive faculties in a person who was previously cognitively normal. Its etiologies include neurodegenerative disorders such as AD and Lewy-body dementia. AD, in particular, is associated with reduced hippocampal volumes. In a metaanalysis of potential neurostructural predictors for the progression from mild cognitive impairment (MCI) to AD, volume reductions in the hippocampus and parahippocampal gyrus were the most consistent predictors of conversion from MCI to AD.
45 One could hypothesize that the smaller hippocampal volumes in chronic CR-PTSD noted in the studies reviewed here would put patients at greater risk for AD.
However, two of the reviewed studies suggest a different interpretation. The sole longitudinal study
39 did not find increased hippocampal atrophy rates in PTSD patients, suggesting that the PTSD disease process does not lead to reduced hippocampal volumes. Accordingly, it is possible that smaller hippocampal volumes pre-dated the traumatic event, in which case reduced hippocampal volume could, in fact, be a risk for PTSD. Indeed, a twin study on CR-PTSD appears to also support this interpretation. Gilbertson et al.
21 compared two types of monozygotic twin pairs: 1) combat veterans with CR-PTSD and their non-combat twins; and 2) combat veterans without PTSD and their non-combat twins. Hippocampal size correlated well between twin brothers; moreover, both the CR-PTSD veterans and their twins had smaller hippocampi than the combat veterans who never developed PTSD. This study, too, suggests that smaller hippocampi may be a risk factor for CR-PTSD. Together, these findings support the hypothesis that reduced hippocampal volumes are a risk factor for PTSD and AD, rather than one causing the other.
Nevertheless, these data are not definitive because other potential mechanisms may play a role. In one study, the hippocampal volumes of recent trauma-exposed individuals (within 1 week) did not differ in those who would subsequently develop PTSD at a 6-month follow-up assessment, as compared with those who would not develop such symptoms.
46 Also, if the hippocampal volumes were entirely determined by genetic predisposition, the Gilbertson et al.
21 data should show hippocampal differences between the CR-PTSD+ and CR-PTSD− veterans mirroring the differences between the non-combat individuals. In fact, whereas the difference in total hippocampal volume was significant between the veteran groups, the difference between the non-veteran groups was not significant, which suggests that an additional environmental factor may play a role in the volumetric differences.
Concerning the negative studies included in this review, two reported no significant results in their respective regions of interest.
29,32 One study
32 found slightly reduced, but nonsignificant, right hippocampal volume reductions in PTSD veterans versus normal controls; however, they did show reduced N-acetylaspartate (NAA) levels, a marker of neuronal integrity, in the right hippocampus nearly meeting significance levels (p=0.06). Importantly, the small sample size (N: 7 PTSD+; N: 7 PTSD−) represents a notable limitation in this study, and the study received a QS rating of 0 for the purposes of this review. Similarly, a second study
29 found no differences between veterans with PTSD and veterans without PTSD in hippocampal or entorhinal cortical volumes, but did find significant bilateral reductions in NAA density (Left: p=0.019; Right: p=0.012) in the PTSD cohort. Notably, in their linear-regression models, further accounting for left hippocampal and entorhinal cortical volumes accounted for 15.3% of incremental variance (p=0.023). Although these two studies failed to report associations between PTSD and reduced hippocampal volumes, they do provide evidence that PTSD effects on hippocampal neuronal integrity represent either a plausible risk factor or potential modifier for subsequent dementia.
Clearly, more longitudinal studies are needed to differentiate between these hypotheses and to determine whether treating PTSD reduces the risk of subsequent dementia. If reduced hippocampal volume is a risk for both, then treating PTSD will not prevent dementia. Clinicians would instead focus on early detection and treatment of dementia in those with PTSD.
Our review has certain limitations. Like all reviews, our results are limited by the “file-drawer” problem, the idea that researchers may not report or publish negative results.
47 Also, we only examined studies that were published in English, which reduces the number of studies meeting our methodological criteria. The imaging methodologies of the studies included (e.g., strength of the MRI field, thickness of structural slices, interrater reliability for morphometry, preprocessing/enhancement of images, and delineation of anatomical landmarks) were not consistent, and, in general, studies were inconsistent with regard to the quality of their confirmation of PTSD diagnosis and exclusion/inclusion criteria, as well as types and severity of both the severity of the experienced trauma and PTSD symptomatology. Moreover, there may be differences between studies in the veterans’ experiences of war and combat-related trauma, such as differences related to the particular combat in which they were involved and the evolution of warfare. Most studies also differed with regard to controlling for associated disorders, such as severe depression and alcohol abuse, both of which are frequently concomitant with PTSD and have been shown to reduce hippocampal volume.
48–52 Some studies were also performed by the same authors over time, which could potentially introduce bias. Certain premorbid factors, such as previous trauma exposure, including both adult and early-life stress, or preexisting psychiatric/neurological disorders, may influence PTSD development
53–55 but were not consistently controlled for in the studies reviewed. Also, this review was unable to control for presence of traumatic brain injury (TBI). Over 40% of returning U.S. Iraqi veterans with mild TBI met PTSD criteria,
56 whereas lower, but still significant, correlations appear between PTSD and severe TBI.
57,58 These associations are significant, as TBI has also been shown to be a risk factor for dementia in two large veteran cohorts.
59,60 Finally, this review focused on studies evaluating veterans with combat-related PTSD, including studies primarily or exclusively using male subjects; therefore, these results may not be directly applicable to female veterans with PTSD or civilian PTSD populations.
Future Research
Whether the chronic PTSD disease process results in reduced hippocampal (or other brain region) volumes, or these reduced volumes represent pre-existing variation, still needs to be investigated. Therefore, there is an urgent need for further studies of trauma, and both volumetric and functional neuroimaging will provide important data. For case–control studies, fully identifying the type and severity of trauma as well as the duration and severity of the PTSD symptoms is paramount. In order to provide significant evidence regarding the neuroanatomical changes associated with PTSD, our recommendations are that future studies be 1) performed longitudinally; 2) consist of two separate matched controls: trauma-exposed and trauma-naïve; 3) consist of multiple MRI acquisitions, preferably pre-trauma, immediately post-trauma, and at subsequent follow-up assessments; 4) account for relevant PTSD risk factors,
53,54 such as the number of previous stressful events or pre-existing anxiety/depression; 5) document the type, duration, and severity of the physical trauma; and 6) provide empirical data on PTSD severity and duration.
To further clarify the relationship between PTSD and dementia, long-term prospective studies that follow trauma-exposed individuals for extended time-frames are required. Also, twin studies would also be informative, as twins discordant for combat exposure should provide compelling evidence regarding whether combat exposure and/or PTSD causes an increased risk of dementia.
Another important research objective would be to determine the effects of timely PTSD treatment methods and subsequent reductions in PTSD symptoms, both duration and severity, and the rates of other disease processes that may be mediated by a chronic PTSD disease course.
Clinically, multiple studies have shown that PTSD may produce long-term negative physical
5 consequences and neurocognitive deficits.
12 Although this review concludes that PTSD is associated with reduced hippocampal volumes, a causative relationship cannot be determined. However, as PTSD has been associated with an increase in vascular risk factors
61 and reduced cognitive ability,
62 it is imperative that proper PTSD treatment regimens be implemented as soon as possible to prevent any further potential damage. In addition to both pharmacological and psychological PTSD therapies, vascular risk factors and relevant behavioral modifications (e.g., increased alcohol or nicotine dependence) should be closely monitored in this population, while preventive measures such as increased physical activity should be stressed.