To the Editor: The complex connectivity of basal ganglia with other cortical and thalamic circuits that guide our behavior, cognition, and movement is still a matter of controversy. However, the past decade brought about the increased insight into the synchronized oscillations between basal ganglia-cortical and thalamo-cortical circuits, as well as the awareness about the potential detrimental effect that any interruptions (including brain infarction) of these oscillations may instigate.
1,2 Small infarctions in the subcortical white- and gray-matter areas are sometimes clinically overlooked and only inadvertently reported on post-hoc neuroimaging.
3–8 Furthermore, even those subcortical lesions that appear to be strategically placed are sometimes without overt clinical signs and with symptoms that come and go despite the presence of fixed cavities on the neuroimaging. (as reviewed in
4). Consequently, a certain incredulity on the part of investigating clinicians, when assessing the retrospective reports by patients and their families about associated symptoms, is common, and their putative impact on cognition and behavior is not always easily argued.
3,4 The converging evidence, however, suggests that such infarcts could affect distant areas/neuronal circuits via a combination of local and remote chemical and electrophysiological effects.
3,4,9–11 We present here the case of intermittent behavioral and cognitive changes associated with the striatocapsular lesion due to infarction. It is hoped that this and similar case reports that detail the plethora of paroxysmal neuropsychiatric symptomatology resulting from lesions in this area will aid improved clinical recognition of subcortical “silent” infarcts.
Case Report
“Mrs. A,” a 50-year-old, right-handed shop-assistant suffered with a sudden onset of transient flushing attacks and hot sweats associated with behavioral and cognitive changes. Sporadic attacks initially appeared in clusters over 1 to 2 days and were reported to last seconds-to-minutes. At work, customers complained that during attacks she would abruptly start to talk nonsense, slur, and/or persevere in her answers. At other times, she would giggle inappropriately while making strange noises, or rhythmically tap and touch items in a disinhibited manner. The attacks did not seem to be associated with any overt precipitating external or internal stressors and would happen indiscriminately of place. Her family and friends all appeared to recognize these events as entirely out of character. Subsequent to a flurry of these episodes, she would usually experience brief transient episodes of depressive symptoms, when she was reported to exhibit retarded, anhedonic, and bradykinesic behavior. On her presentation to hospital several months after onset, a number of such short and circumscribed episodes of confusion, followed by several days of increased emotional lability and hypersomnia, were described. Further detailed questioning revealed that strong feelings of undirected panic and doom were occasionally also experienced during her clusters, as were instances of palpitations and chest pain. Decreased libido was also noted. No photophobia, vomiting, or visual disturbance were reported. She was otherwise physically intact, with no other overt abnormalities or psychiatric symptoms registered between the episodes.
The diary kept by her family provides additional insight into some of the attacks: “Found Mum sitting on the kitchen floor in front of the washing machine; she was staring, not knowing which button to press, did not move…. (Entry 2:). Mum is all red in her face again, she is swallowing with her mouth, looks all frightened only to burst laughing later on, constantly fidgeting ….(Entry 3:) both her eyes and her head turned to the right side, her face twitching; this lasted for several minutes, and later she started again to laugh, left hand fingers twitching. (Entry 4:) several short episodes of being flushed in face. …found her in a strange stooping posture, like an old lady. (Entry 5:) she was in front of the TV, TV not on, she was giggling. (sic)” Other entries read: ‘out walking, all well, then that strange “clown” smile again, she started whistling, took me over and started hopping along like a school girl. .. later she wandered off in the cafeteria, randomly picking dirty leftover cups and then making repeated moves as though drinking from them. Confused, giggling. Later did not remember she did it… (sic)”
Hospital investigations revealed hypertension, hypercholesterolaemia, and left-ventricular diastolic dysfunction. Positive family history of diabetes and cardiovascular history were also reported. There was no significant past history of affective or anxiety disorders. Endocrinological examination showed FSH, LH, and estradiol at perimenopausal status levels; however HRT commencement did not appear to help. “Ictal” EEG (recorded during one of the episodes) showed no overt epileptiform changes. Increased heart rate and hyperventilation were also noted during some of the hospital episodes. Her cognitive examination revealed patchy impairment, and she scored 80/100 on Addenbrookes' Cognitive Examination (ACE; MMSE was 27/30);
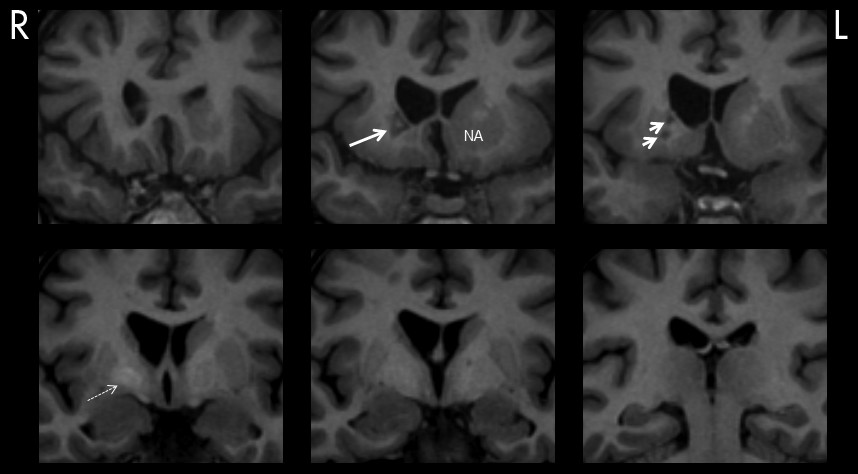
Figure 1. Neuroimaging showed a striking striatocapsular lesion suggestive of old infarct(s); (
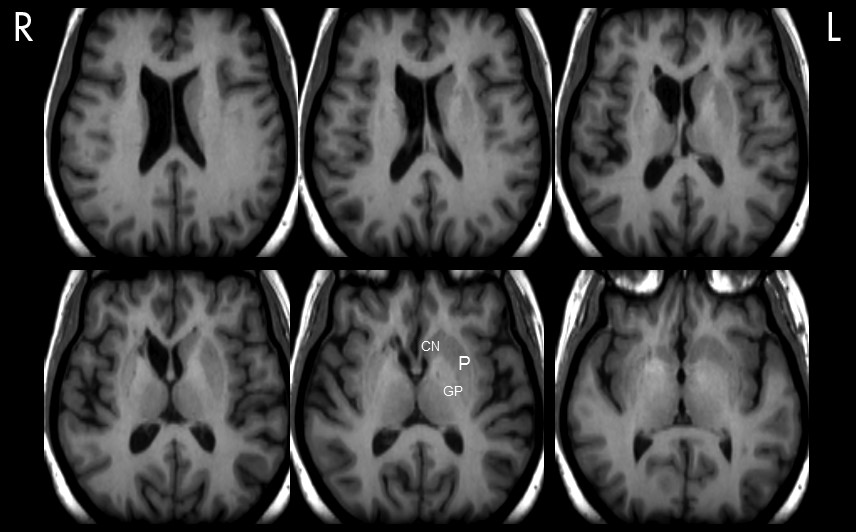
Figure 2,
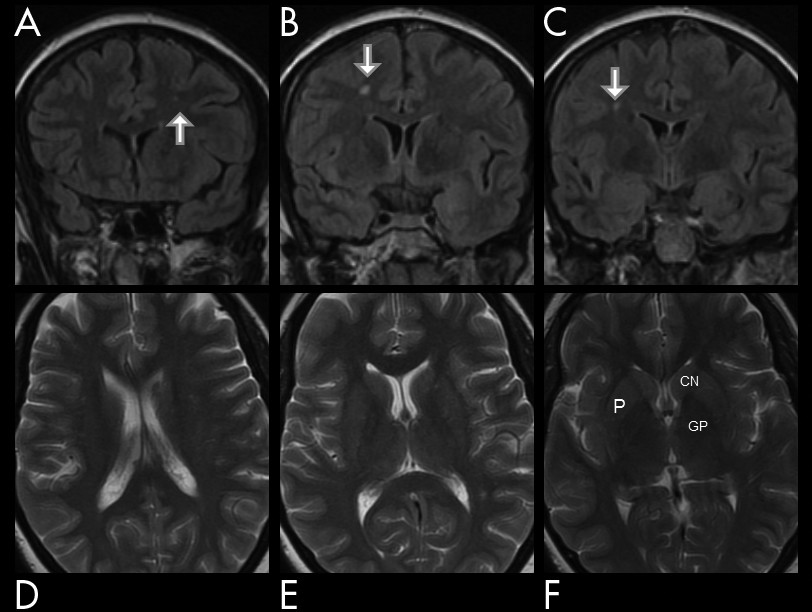
Figure 3). The earlier MRI scan taken after non-migrainous headache complaints, which shortly predated the reported onset of the attacks, was, on the other hand, reported normal (see
Figure 4). Subsequently, an aggressive cardiovascular risk factors’ management was undertaken, and, on follow-up 18 months later in the outpatient clinic, she was found neuropsychiatrically intact, with only scarce and sporadic symptoms reported.
Discussion
Striatocapsular infarcts are usually defined as large subcortical infarcts in the territory of the lenticulostriate arteries that sometimes can extend into the territory of adjacent deep perforators.
3 These arteries arise at an acute angle from the middle cerebral artery to form curves and end in certain parts of the basal ganglia and internal capsule.
12 The controversy about the etiology of striatocapsular infarcts is still alive, with some supporting a “lacunar hypothesis,” whereas others define them as ischemic infarcts.
3 Frequent hemorrhagic transformation (74%) is taken to suggest cardioembolic genesis in some of those infarcts (see
Figure 2).
3In the case presented here, it is ambiguous whether the final lesion was a result of one or several temporally and etiologically independent events. The clinical history would, however, suggest several distinct TIA-like events. Panic-like attacks with autonomic instability, acute ipsilateral eye and head deviation, posturing, and altered consciousness were all noted, but the prevailing elements of the clinical picture, however, were the associated intermittent cognitive and behavioral abnormalities. The striking mix of hypo- (lack of initiative for action and speech, apathy, abulia, inertia) and hyperactivity (decreased ability to direct sustained attention to tasks, stereotypies, disinhibition, and agitation) seen in this case was previously described in patients with caudate infarcts.
6–8 In a study of long-term cognitive impairment in patients with subcortical lesions, those with involvement of caudate nucleus had lower scores on the MMSE.
13 A subgroup of patients showed further deterioration in their test scores despite no new strokes during the 1–2 year follow-up. In our case, cognitive examination revealed patchy impairment in several cognitive/cortical areas (e.g., fronto-temporo-parietal) leading to decreased overall score on the ACE. The most affected were phonetic verbal fluency, verbal memory, and free recall—the similar cognitive functions previously reported as affected in deep brain studies (DBS) targeting basal ganglia.
13 Bokura and Robinson (1997) recorded depression in patients with caudate stroke acutely, but did not find it on follow-up.
14 This acute clinical pattern was also present in Mrs. A's case.
The cortical symptoms in subcortical strokes are thought to result from a disruption of connecting fibers between various cortical centers or functional de-activation of cortical areas by the subcortical lesion (diaschisis, or disconnection syndrome).
3,4,9 The possibility of the temporary ischemic penumbra or selective neuronal loss of the cortex due to prolonged ischemia was also proposed.
3 Correspondingly, the cognitive and behavioral changes here can be attributed to loss of function of cortical zones caused by loss of striatal efferent projections from the caudate nucleus. This nucleus participates in a number of cortico-striato-pallido-nigral-thalamic cortical circuits, and the prefrontal, preorbitofrontal, anterior cingulum, and temporal lobe limbic cortex are all included in the circuitry.
4–7 In patients with striatocapsular infarcts, functional deactivation and associated reduced cerebral blood flow was shown in the insula, lateral prefrontal, and primary sensorimotor corticies, the thalamus, cerebral peduncle, and the contralateral cerebellum.
3The caput caudatum (e.g., superio-medial part) has been implicated in the control of language and selection/inhibition, and perseveration was described during its simulation.
15 Similarly, it is perhaps conceivable that experienced anhedonia and loss of libido in our case could be explained by interruption of the ventral tegmental area of the brain reward circuit that projects dopaminergic fibers to the NA and globus pallidus (
Figure 2,
Figure 3).
16 Motor weakness was also reported in patients with larger caudate infarcts, where it was explained by interruption of striato-pontine fibers projecting in the anterior limb of the interior capsule and by involvement of the putamen.
4Episodic emotional lability (e.g., pathological laughing) was another salient feature recorded in the diary kept by the patient’s family. Post-stroke emotional lability (PSEL) was previously associated with anterior cortical, frontal lobe, and lenticulocapsular lesions.
17,18 Lesions that involved the dorsal globus pallidus were shown as particularly prone to PSEL. As shown in
Figure 2, neuroimaging in our case showed hyperintensity of this nucleus, suggestive of previous microhemorrhage. Moreover, cerebral microbleeds, which are traditionally considered clinically silent and can result in focal deposits of hemosiderin, have recently been implicated in the genesis of PSEL, as well as cognitive impairments in subcortical vascular dementias.
17Perhaps analogous to the observed clinical picture in our patient, reproducible sensations of flushing, hyperventilation, fear, panic, and sweating were also recorded during DBS of the anterior limb of the internal capsule in the NA region.
19,20 Okun and others (2007) reported that, during stimulation of the anterior limb, the patient felt palpitations in his chest and talked about feeling of an impending sense of doom.
19 Stimulation in the nearby NA area, conversely, caused feelings of giddiness, of wanting to laugh, and feelings of euphoria. So far, the NA region had not been considered to play a role in the pathophysiology of panic attacks. Classically, it was solely amygdalofugal and hypothalamic pathways, as well as amygdalar connections with the locus ceruleus and the periaqueductal gray matter, thalamus, and frontal cortex that were understood to produce hormonal, autonomic, and behavioral changes characteristic of panic attack.
20 Notwithstanding this earlier construct, it has been argued that DBS of NA may also activate limbic and autonomic networks via hypothalamic and/or autonomic fibers in the anterior limb, thus mimicking panic attack.
20 Affective manipulation (e.g., both worsening and improvement of mood) were also achieved with DBS of the NA region. This effect was reported as area-specific and voltage-dependent.
19,20Current evidence lends some support to the concept that infarction may have net excitatory and/or inhibitory effects through its regional chemical and electrophysiological mechanisms.
3,10,11,21 The presence of a fixed cavity in the internal capsule of the NA region in our patient probably suggests this as an area of leading impact. In the past, the hemodynamic mechanisms in penetrating arterial territories were proposed as culprits behind symptoms in the “capsular warning syndrome.”
11 Molecular mechanisms and metabolic dysfunction, such as peri-infarct depolarizations, are thought to affect adjacent long tracts in deep white matter. Interestingly, the gray- and white-matter oligodendrocytes, crucial for axonal salutatory conduction and transmission speed, were shown equally vulnerable as neurons to ischemia in vivo.
10 As reviewed by Dewar and others (2003), rapid morphologic changes in subcortical white-matter oligodendrocytes were reported in response to permanent middle cerebral artery occlusion in the rat. During the transient global ischemia, oligodendrocytes showed greater vulnerability than neurons in the cortex and thalamus, which appeared to sustain only minimal damage.
10 In contrast, the susceptibility of oligodendrocytes was shown to be similar to severity of neuronal damage in the striatum.
10 Drawing on these previously asserted pathophysiological mechanisms, we here argue that paroxysmal episodes in our case report may actually represent a covert prolonged crescendo warning of the longer-standing striatopallidocapsular ischemia, affecting both subcortical white and gray matter.
The exact etiology of these clusters is far from clear. It is perhaps conceivable that something like chemical or other covert electrophysiological or vascular factors could have led to a build-up of local tissues' vulnerability, to the point when a cluster of episodes would be instigated. However, a chance temporal association between the infarct(s) and the behavioral changes described cannot be excluded with certainty. Nor can we with certainty exclude the potential dissociative, psychogenic, or indeed epileptic etiology of the reported paroxysmal attacks. However, we reason that the plethora of cortical symptoms in our patient with subcortical basal ganglia infarction points to a disrupted activity of the affected area, potentially further causing a disruptive “ripple” effect on the local and distant frontal networks oscillations.
3,4,9,11