To the Editor: Yokukansan, a traditional Asian herbal medicine, is reported to be safe and effective for behavioral and psychological symptoms of dementia in randomized, controlled trials, and is widely prescribed for patients with dementia in Japan.
1 A recent open-label study indicates that adjunctive yokukansan administration in treatment-resistant schizophrenia improved the positive and negative symptoms of schizophrenia.
2 In this report, we present a case of schizophrenia in which adjunctive yokukansan treatment dramatically improved severe cognitive dysfunction.
Case Report
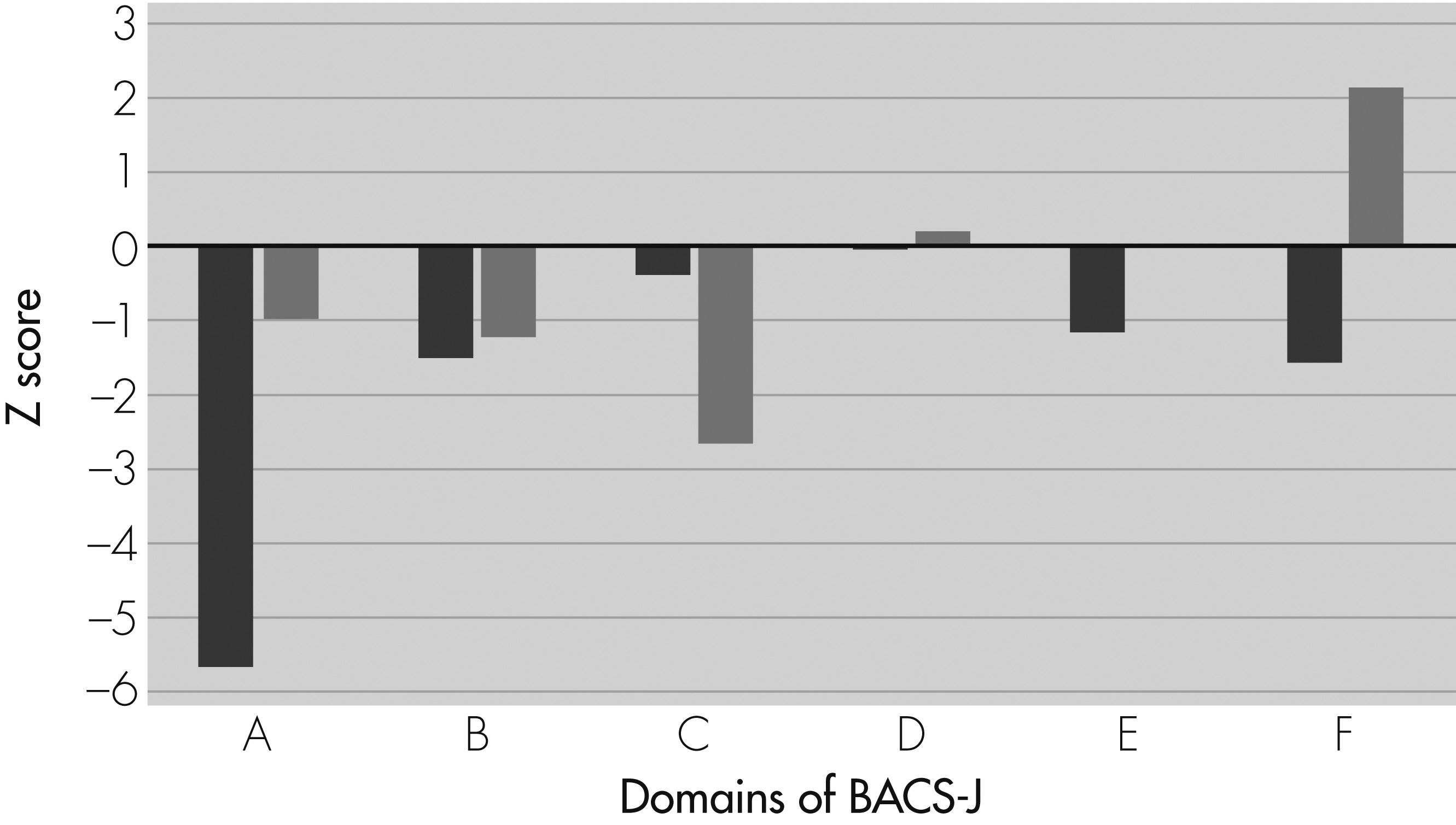
The patient is a 32-year-old Japanese woman with schizophrenia. When she was 20 years old, she presented at a mental health service with auditory hallucinations and delusions of being observed. Although she was treated with 25 mg/day olanzapine and 72 mg/day perospirone, her symptoms improved only partially. In August 2008, she complained that she could not understand the content of TV programs and books at all. The Brief Assessment of Cognition in Schizophrenia, Japanese Version (BACS–J) showed that her cognitive functions were widely impaired, especially verbal memory (Z score = −5.69), attention and processing speed (Z score = −1.16), and executive function (Z score = −1.57). The total Positive and Negative Symptom Scale (PANSS) score at the time was 92.
In September 2008, olanzapine and perospirone were supplemented with yokukansan (5.0 g/day) taken twice a day. Three months later, she gradually began to understand TV dramas, and she was better able to follow a baking recipe for a cake. Twenty-nine months after the first prescription of yokukansan, we administered the BACS–J again. The scores on the verbal memory (Z score = −0.96), attention and processing speed (Z score = +0.04), and executive function (Z score = +2.13) were robustly improved (
Figure 1), but the total PANSS score was 89. Obviously, adverse effects were not seen after administration of yokukansan.
In the present case, cognitive functions in daily life and BACS–J score were markedly improved after administration of yokukansan. Disturbed cognitive functions in schizophrenia are supposed to be associated with dysregulation of serotonin (5-HT) and glutamate transmission.
Several studies demonstrated that yokukansan had a partial agonistic effect on 5-HT
1A receptors. In a study to evaluate the binding and intrinsic activity of extracts of yokukansan on 5-HT
1A receptors, Uncaria hook, one of its constituent herbs, showed 50% activity of a full agonist.
3Repeated treatment with yokukansan significantly inhibited the 5-HT
2A receptor agonist-induced head-twitch response and decreased expression of 5-HT
2A receptors in the prefrontal cortex of mice.
4On the other hand, the effects of yokukansan on the glutamate system have also been reported. Yokukansan ameliorated the decrease of glutamate uptake and glutamate-aspartate transporter in astrocytes induced by thiamine deficiency in a dose-dependent manner.
5 Furthermore, extracellular glutamate increases in zinc-deficient rats was also attenuated by administration of yokukansan.
Therefore, yokukansan mediates 5-HT transmission as a partial agonist on 5-HT1A receptors and a modulator of 5-HT2A receptors and ameliorates the disturbance of glutamate transmission in pathological conditions. The present case suggests that adjunctive yokukansan treatment might be effective for cognitive dysfunctions in schizophrenia. Further clinical and pharmacological researches are required.