There is evidence that ischemic stroke is intimately linked to inflammatory processes, and proinflammatory cytokines appear to be involved in the pathogenesis of brain ischemia.
7,10–12 Moreover, cytokines have been extensively proposed as key factors in the modulation of mood and other psychiatric disorders.
13–21 Accordingly, very recent data have demonstrated that cytokine-dependent T-cell dysfunction might have a role in depressive disorders and that polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response.
22 Also, the hypothesis that proinflammatory cytokines may play a central role as mediators of mood and mood-related disorders even in patients with stroke has been previously formulated.
23,24 However, only a limited number of reports suggest a role of neuroinflammation on post-stroke affective phenomenology.
13,25–27The attention of the literature has been recently focused on interleukin 6 (IL−6), a pleiotropic cytokine involved in inflammatory processes and able to induce acute inflammatory responses, as does interleukin 1 (IL−1).
28 Although a clear picture of the role of IL−6 in stroke has not yet been elucidated, increased IL−6 production after cerebral ischemia has been described in several clinical studies,
10,26 and elevated levels of IL−6 have been correlated with severity and clinical outcomes of stroke.
24,26 All the above studies show heterogeneous findings due to different stroke populations and the inclusion and exclusion criteria adopted. Moreover, patient and control groups were often not matched for all demographic variables, and patients with depression were under varying exposure to antidepressant therapy in terms of dosage and duration. Another important limitation is the presence of confounding factors able to influence cytokine production, including immunologically-mediated disorders, chronic inflammatory diseases, cancer, etc. Finally, IL−6 levels were mostly investigated during different phases of the stroke (acute, subacute, or chronic).
24,29The relevance of IL−6 as a prognostic marker of post-stroke clinical outcome and the potential effect of depression on stroke recovery support the need to investigate the hypothesis that IL−6 production, associated with brain ischemic lesion, contributes to the pathogenesis of stroke-related mood, cognitive, and functional disorders, starting from the very acute phase.
Thus, the main aim of our study was to investigate the relationships between IL−6 serum level and severity of neuropsychiatric and functional symptoms, impaired neuropsychological functions, and clinical neurological outcomes in patients with acute ischemic stroke. We have also examined which individual symptoms of depression are associated with IL−6 levels.
Methods
All data were collected in the Stroke Unit of the Sant’Andrea Hospital in Rome, Italy; 48 consecutive patients admitted to the hospital for a first-ever stroke were enrolled in the study. Only patients without significant clinical factors that promote inflammation were selected. Particular attention was given to exclude patients suffering from those diseases that are known to be associated to increased IL−6 production that may be very common in elderly people, such as autoimmune diseases (e.g., Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus), infections, history of previous stroke, pre-stroke psychiatric and neurological disorders (e.g., pre-stroke depression, Alzheimer’s disease), and cancer. Patients who underwent surgical procedures determining chronic inflammatory reaction (see exclusion criteria) were also excluded. In particular, the inclusion criteria were the following: 1) first acute ischemic stroke diagnosis according to National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) criteria;
30 2) vision and hearing sufficient for compliance with testing procedures. Exclusion criteria included the following: 1) severe or moderate cognitive deficit as evaluated by Mini-Mental State Exam (MMSE)
31 score <17; 2) aphasia with moderate or severe impairment of language comprehension precluding both the interview and the administration of the questionnaires; 3) previous history of head trauma or other brain diseases; 4) major medical illnesses (e.g., diabetes; obstructive pulmonary disease or asthma; hematologic disorders; clinically significant and unstable active gastrointestinal, renal, hepatic, endocrine, or cardiovascular disorders); 5) history or evidence of any clinically important autoimmune disease or disorder of the immune system; 6) history of cancer within the last 5 years; 7) clinically important infection within the last 30 days (e.g., chronic persistent or acute infection, such as bronchitis or urinary tract infection); 8) implant of carotid or coronary stent or other major surgical interventions; 9) known or suspected lifetime history of alcoholism or drug dependence and abuse; 10) use of anti-inflammatory drugs within the last 30 days (e.g., corticosteroids or nonsteroidal anti-inflammatory drugs); 11) history of psychiatric treatment (e.g., antidepressants, antipsychotics, mood stabilizers, benzodiazepines).
At the admission into the Stroke Unit, an expert neurologist performed a full neurological evaluation of the patients. The ischemic lesion was assessed and confirmed by both the clinical examination and computed tomography (CT) or magnetic resonance imaging (MRI).
In order to evaluate biological markers during the acute phase, one blood sample was obtained about 72 (±12) hours after the acute stroke onset, in the early morning, with the patient fasting. We selected this time-point because several studies have reported that IL−6 levels peak at 3 days after an acute event.
32–34 Moreover, in all acute patients admitted to the Stroke Unit at this time-point, it was possible to perform the blood sampling for measurement of the IL−6 levels. Thus, the blood sample collection at 72 hours after the neurological stroke symptom onset may be a good clinical outcome (both from a biological and practical point of view) for this inflammatory marker.
All included patients underwent neuropsychiatric, neuropsychological, functional, and neurological assessments, carried out 3 days (about 72 hours) after the onset of the neurological symptoms of the acute stroke, when the patient was stabilized and we were able to perform the evaluations. Trained specialists (one psychiatrist for the psychiatric diagnoses, three neurologists for the neurological assessment, and two neuropsychologists for the neuropsychological and psychiatric rating scales) who were blind to the aims of the study performed the testing. Evaluation of interrater reliability in this study was in the excellent-to-good range for all scales used, with intraclass correlations ranging from 0.80 to 0.93.
The study was approved by the ethical committees of the IRCCS “Santa Lucia” Foundation and “Sant’Andrea” Hospital, and, in accordance with the Helsinki Declaration, each subject signed an informed consent form before enrollment.
Neuropsychiatric Assessment
All patients underwent a structured psychiatric interview (SCID-P)
35 for the identification of mental disorders, according to the Diagnostic and Statistical Manual of Mental Disorders 4th Edition–Text Revision (DSM–IV-TR);
36 in particular, the presence of Major Depressive Disorder (MDD) and Minor Depressive Disorder (MiDD) was investigated. The DSM–IV-TR diagnostic criteria both for symptoms (criterion A) and functioning (criterion C) were observed to determine the diagnosis of major and minor depression, although the part of criterion A based on symptoms present during the same 2-week period could not be assessed because of the scheduling of evaluations. In particular, for this part of the criterion A, we initially assessed the first 3 days after the stroke onset, and eventually we confirmed the presence of symptoms for the same 6-day period, from admission to the stroke unit to the last clinical evaluation before discharge from the hospital. Thus, the symptom-duration part of the A criterion was modified, in this study on acute stroke inpatients, from the same 2-week period to the same 6-day period. The SCID–P was also used to measure the frequency of the nine individual symptoms of MDD, as indicated by the symptom criteria (in criterion A) of the DSM–IV-TR, in our stroke patients with and without depression.
Depressive symptom severity was evaluated with the Hamilton Rating Scale for Depression (Ham-D),
37 a 17-item inventory composed of psychological (PSY) and somatic (SOM) subscores that together contribute to the total score. The PSY subscale consisted of 6 questions about depressed mood, guilt, suicide, work, loss of interest, anxiety, and insight; the SOM subscale consisted of 11 questions regarding insomnia (initial, middle, and delayed), retardation and agitation, somatic anxiety, somatic gastrointestinal symptoms, general somatic symptoms, genital somatic symptoms, loss of weight, and hypochondriasis.
Severity of anxiety symptoms was measured with the Hamilton Rating Scale for Anxiety (Ham-A).
38Neuropsychological Assessment
Global cognitive functioning was evaluated with the Mini-Mental State Exam (MMSE).
30 To assess performance in specific cognitive domains, the patients were administered the Mental Deterioration Battery (MDB),
39 a standardized and validated neuropsychological battery including the Rey's 15-word test: Immediate Recall (RIR), Delayed Recall (RDR), and Word Recognition (RWR), to evaluate short- and long-term verbal memory; the Copy Drawings (CD) and Copy Drawings with Landmarks (CDL), to evaluate simple and constructional praxis, and the Stroop Word–Color Test (SWCT)
40 for the evaluation of executive functions of attention-shifting and control.
Functional and Neurological Assessment
We used the Barthel Index (BI)
41 to evaluate functional abilities and the National Institutes of Health Stroke Scale (NIHSS)
42 for the quantitative evaluation of stroke-induced neurological deficit during the acute phase. In particular, the NIHSS is a 15-item neurologic examination stroke scale useful for measuring stroke severity and predicting both short- and long-term outcomes of stroke patients by evaluating the effect of acute cerebral infarction on level of consciousness, language, neglect, visual-field loss, extraocular movement, motor strength, ataxia, dysarthria, and sensory loss. Ratings for each item are scored with 3-to-5 measures, with 0 as normal, and there is an allowance for untestable items.
IL−6 Measurement in Serum
Serum was obtained from all patients at 72 hours after the onset of the acute ischemic stroke symptoms by centrifugation of clotted blood samples; aliquots were stored at −80°C until cytokine assay. IL−6 levels were determined with Quantikine HS High Sensitivity ELISA for Human IL−6 (R&D Systems, Minneapolis, MN). The sensitivity of the assay was 0.039 pg/ml.
Statistical Analysis
Univariate analyses of variance (ANOVAs) followed by Fisher’s protected least significant deviance (PLSD) were performed to assess differences in IL−6 mean values and other continuous variables (the dependent variables) among patients with MDD, MiDD, and no depression (NODEP); diagnostic groups were considered as the independent variable. Fisher’s PLSD test was used for pairwise comparisons in post-hoc analyses.
A series of nine t-tests was performed in order to compare IL−6 values between the two groups of people with and without each of the nine depressive symptoms identified by the SCID-P. In these analyses the level of statistical significance was set at p <0.05 after Bonferroni correction for multiple comparisons (α <0.05/9 number of comparisons − α <0.006).
The predictors of IL−6 levels were determined by means of a series of stepwise multiple-regression analyses, using a forward procedure and an F to enter at 4. Pre-selection of variables to include in the stepwise regression models was done by using correlation analyses and Fisher’s r-to-z transformation in order to determine the significance of correlations for continuous variables. In the stepwise multivariate models, only variables with p<0.05 in the pre-selection analyses were included. The level of statistical significance in these multivariate analyses was set at p <0.05.
Results
Sociodemographic and clinical characteristics of 48 patients with first-ever stroke are summarized in
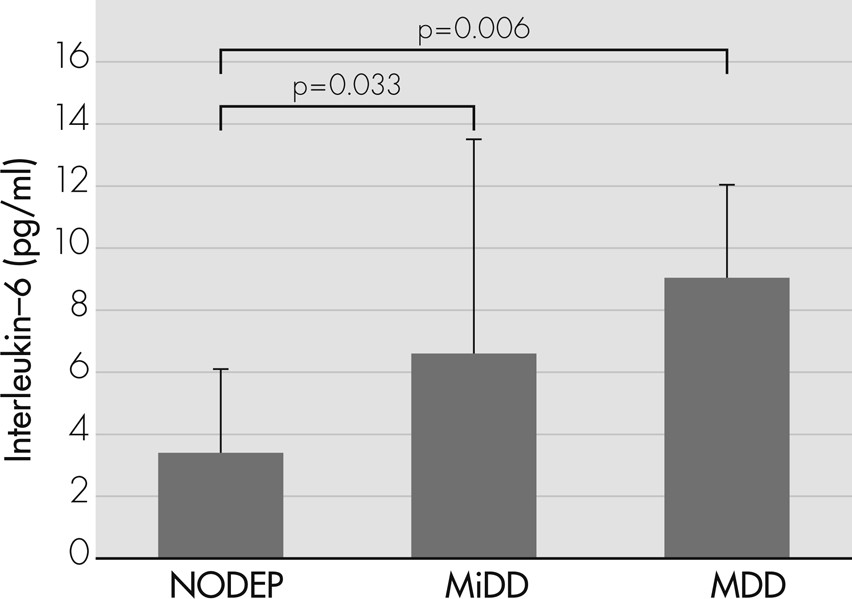
Table 1. The occurrence of depressive disorders was 41.7%. In particular, 6 patients (12.5%) were diagnosed as having MDD; 14 patients (29.2%) had MiDD; and 28 patients (58.3%) did not show mood disorders (NODEP).
The analysis of variance (ANOVA) showed an increased progression of IL−6 levels (
F[2, 45]=5.318; p=0.008) from NODEP (mean [standard deviation {SD}]: 3.39 [2.69]) to MiDD (6.55 [6.94]), to MDD (9.05 [3.01]). In particular, post-hoc analyses revealed that MDD (p=0.006) and MiDD (p=0.033) patients had significantly higher IL−6 levels than NODEP patients (
Figure 1).
Table 2 shows differences on IL−6 values among patients with or without each depressive symptom identified by the SCID−P; only patients with at least one depressive symptom among the following: loss of interest, loss of weight or appetite, and insomnia, had significantly higher IL−6 values than those without that depressive symptom.
Table 3 shows raw correlations between IL−6 values and cognitive, neuropsychiatric, and clinical variables; IL−6 levels were positively correlated with Ham-D (PSY, SOM, and Total score) and NIHSS scores, and negatively correlated with RWR Correct Recognitions and BI score.
To clarify which variables were independently correlated with the levels of IL−6, we performed multiple stepwise regression analyses. The preselected variables related (p
<0.05) to the dependent variable in the univariate correlation analyses (see
Table 3) were considered as independent variables. Results of stepwise multiple regression analyses are shown in
Table 4: significant predictors of the IL−6 values were Ham-D, SOM, and NIHSS scores. The resulting equation was highly significant (
F[2, 45]=22.095; p <0.0001) and explained 49.5% (r
2) of the overall variance in IL−6 values. In particular, more severe somatic symptoms of depression and higher degree of stroke severity predicted higher IL−6 values in patients with acute ischemic stroke.
Discussion
In the present study, we investigated the relationship between IL−6 serum levels and psychiatric, neurological, cognitive, and functional features during the acute phase of ischemic stroke.
We found that IL−6 levels were increased in patients with depressive disorders, particularly in those with MDD. Furthermore, IL−6 levels were associated with severity of the apathetic−amotivational and somatic symptoms of depression and the neurological symptoms of stroke. Noteworthy, unlike other studies focused on IL−6, in our sample we included only patients without history of possible confounders; thus it is unlikely that our results were affected by different external factors able to promote interleukin production independently of stroke and stroke-related phenomenology.
Despite the fact that pathophysiological consequences of acute ischemic stroke are still not very well understood, several findings suggest that neuroinflammatory mechanisms play a crucial role in ischemic injury, and the imbalance between pro-inflammatory and anti-inflammatory agents can result in worsened neurological outcomes
43,44 and cognitive and neuropsychiatric disorders.
2,9,24,26,27 Among pro-inflammatory agents, IL−6 has been proposed as an important mediator of stroke-induced immunological/inflammatory reaction. Indeed, elevated IL−6 values after cerebral ischemia seem to be associated with higher stroke severity and worse stroke outcomes.
8,10,44The relationship between IL−6 and stroke severity also appeared in our data. In particular, our study showed that higher IL−6 values are independently associated with higher degree of clinical severity during the acute phase of ischemic stroke. These findings are consistent with other studies showing that plasma and cerebrospinal cytokine fluid levels are related to clinical worsening of patients with acute ischemic stroke.
43 For instance, in the study of Basic Kes and colleagues, an association between inflammatory parameters (IL−6, IL−10, and TNF-α), greater neurological deficit (evaluated by the NIHSS), and greater degree of patient disability (assessed with BI and modified Rankin Scale) was found in stroke patients at admission to the hospital compared with controls.
10 Also, Yan and colleagues, in a study focused on the levels of cytokines up to 3 weeks after stroke, found a relationship between IL−6 levels and stroke severity, especially up to 1 week after the ischemic event.
28 Worthy of note is that whereas most of these studies exploring IL−6 production after ischemic stroke did not exclude potential confounders that often appear after stroke and are associated with both higher levels of inflammatory markers and poor stroke outcomes, independently of other factors,
8,44 we carefully selected our sample of patients in order to avoid all potential factors that affect inflammatory processes. Thus, we can assume that, in our work, the pro-inflammatory cytokine IL−6 is strongly associated with the ischemic brain damage occurring in the acute phase of stroke.
In several studies, changes in cytokine levels have been hypothesized to be associated with the etiology of post-stroke depression (PSD). Our research group suggested that pro-inflammatory cytokines may play a central role as mediators of mood and mood-related disorders, specifically in patients with stroke.
24 We based our hypothesis on the evidence that pro-inflammatory cytokines and ischemic stroke were strongly related and interleukins were correlated with the existence of PSD. Most recently, Yang et al. found that, on Day 1 after stroke, patients diagnosed with PSD have increased serum levels of IL−6 and TNF-α.
29 Finally, Su et al., evaluating cytokine changes over 1 year in patients with ischemic stroke, found a significant increase of cytokines in patients with PSD, confirming the hypothesis that PSD is associated with increased inflammation and that immune dysregulation leading to changes in cytokine levels may be involved in the etiology of PSD.
27 Unfortunately, these previous studies do not clarify the relationship between the complex clinical picture of stroke phenomenology and inflammatory markers such us IL−6. In our study, we clearly identified that, in acute stroke patients, IL−6 production is most closely related to specific symptoms of depression and specific neurological symptoms only, and this is the real innovation of the present findings.
In the literature, only one study described an association between high IL−6 levels and somatic symptoms of depression. In this study, Stewart et al. investigated the association between depressive symptoms and inflammatory markers in 263 healthy adults for up to 6 years; the authors found that the somatic/vegetative symptoms of depression, assessed in the Beck Depression Inventory−II, were predictors of IL−6 changes over 6 years.
45 Thus, to our knowledge, we are the first to describe the relationship between IL−6 and somatic symptoms of depression in the acute phase of stroke. Interestingly, IL−6 was higher in patients with loss of interest, loss of weight or appetite, and insomnia. Although loss of interest (the nuclear symptoms of the apathetic−amotivation syndrome) is one of the two core symptoms of depression along with sad mood, and its association with IL−6 peripheral levels can be part of the strong relationship between inflammation and PSD, loss of weight or appetite and insomnia are somatic symptoms of depression that deserve further explanation. Although IL−6 is mainly identified as an immune-modulatory cytokine with important pro-inflammatory effects,
46 several studies in animal models have found that IL−6 also plays an important role in metabolic pathways. Indeed, mice treated with peripheral IL−6 show loss of weight, increased energy expenditure, and reduced food intake.
47 Moreover, in humans, IL−6 was demonstrated to be associated with weight loss and cachexia in patients with cancer.
48 In sleep disorders, the relationship between IL−6 and sleep is documented both in people with sleep disturbances and in healthy subjects.
49 In particular, people with obstructive sleep apnea who experience fatigue, excessive daytime sleepiness, and narcolepsy have higher levels of IL−6 than normal subjects.
50 Similarly, in healthy people, morning IL−6 levels are positively related to fragmented sleep and wakefulness and inversely correlated with deep sleep and sleep efficiency.
51 Some authors have postulated that IL−6, by stimulating the activity of the hypothalamic-pituitary-adrenal axis, could be involved in the onset of depressive symptoms, insomnia, and alteration of sleep architecture in aging people.
50 Thus, considering the central role played by IL−6 in the regulation of appetite, energy expenditure, body composition, and quality of sleep, we can assume that the overproduction of IL−6 during the early phase of cerebral ischemia might lead to the development of these important somatic symptoms, such as loss of appetite and sleep disturbances, which affect the quality of life of patients and their outcomes after stroke.
The present study has some limitations: 1) the sample of stroke patients is not very large; indeed, 48 patients with acute ischemic stroke were recruited and assessed. However, this is also a strength of the study, because we aimed to recruit patients without any possible confounding factors that might influence the inflammatory process, therefore including a very homogeneous sample of stroke patients; 2) the assessment of all patients might have been influenced by the environment or external stimuli because, during the hospitalization, the patients were treated in a stroke unit that cares for patients during the acute phase. In reality, this problem is common for all patients assessed during the very acute phase of the illness, thereby reflecting the real clinical world of this issue; 3) for the mood-disorder diagnosis, we could not respect the DSM-IV−TR clinical criterion based on duration of symptoms (i.e., at least 14 days) because the mean hospitalization duration in our stroke unit is 6 days. Thus, we opted to reduce the symptom-duration criterion of minor and major depression diagnosis from 2 weeks to 6 days; 4) the blood sample was performed only one time, at 72 hours after the onset of ischemic stroke, and we may have missed possible fluctuations in IL−6 levels and their relationships with the severity of the clinical phenomenology of stroke. Several studies in the literature, however, described IL−6 peak levels at 3 days after the acute event;
32–34 5) finally, during the acute phase, both the extent of brain damage
52 and the social and psychological stressors associated with stroke
53 may be causal factors for PSD, and the weight of these two individual factors should be best clarified in future studies. Furthermore, we did not investigate the relationships among IL−6 secretion, somatic symptoms of depression, and stroke severity during the subacute and chronic stroke phases, when the inflammatory process may be less evident, and residual depressive symptoms may be less dependent on cytokine release.
The strengths of our study include a thorough evaluation of the complex picture of depressive disorders and symptoms and other neuropsychiatric and cognitive features by means of specific and validated scales and diagnostic criteria. Furthermore, in our sample we recruited only first-ever acute ischemic stroke patients without significant clinical history of medical conditions able to influence IL−6 production or promote inflammatory processes.