Research using biological evidence of underlying Alzheimer’s disease (AD) neuropathology reports both over- and under-diagnosis of possible or probable AD dementia, even when expert clinicians use specific diagnostic criteria. Autopsy reports have shown that a significant number of patients diagnosed clinically by dementia specialists as having probable AD in fact lack AD pathology.
1,2 For example, a recent study comparing expert clinical diagnoses for possible or probable AD and neuropathology at autopsy using the National Alzheimer’s Coordinating Center database found positive predictive values from 57.6% to 83.3%, with sensitivities from 70.9% to 87.3%, and specificities from 44.3% to 70.8% depending upon the stringency of neuropathological criteria used.
2To improve diagnostic accuracy, the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria
3 were revised in 2011 by the National Institute of Aging and the Alzheimer’s Association (NIA/AA), where clinical diagnoses can be made with or without supporting biomarker evidence for AD.
4,5 These biomarkers, as stated in the criteria, are meant primarily for research purposes and include those that assess beta-amyloid (Aβ) pathology (measuring cerebrospinal fluid [CSF], Aβ
42, or amyloid plaque on positron emission tomography [PET] neuroimaging), or those reflecting the effects of neurodegeneration (measuring CSF-tau, atrophy on magnetic resonance imaging [MRI], or metabolism using
18F-fluorodeoxyglucose PET).
Florbetapir (
18F-AV−45, FBP) is a PET ligand for the estimation of Aβ neuritic plaque density, and has been commercially available in the United States since 2012. The in vivo detection of Aβ neuritic plaque density in cortical gray matter with FBP-PET has been shown to correlate with the presence and density of Aβ plaques detected pathologically postmortem using Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria where zero to sparse plaques are inconsistent with AD, and moderate to frequent plaques are consistent with AD.
6,7 A standardized uptake value ratio (SUVR) cut-off of 1.1 has been proposed for quantitative determination of positive or negative scans.
6,7 FBP-PET has been validated both by autopsy pathology in end-of-life patients
6,7 and also in earlier stage patients as compared with results from the
11C-labeled amyloid-PET ligand, Pittsburgh compound B (PiB).
8 When compared with postmortem histological diagnosis, FBP-PET showed 3% false-negative and 0% false-positive rates.
7In this study of a subgroup of patients with a clinical diagnosis (using NINCDS-ADRDA criteria) of mild or moderate probable AD dementia who were participating in a treatment trial, we investigated whether baseline characteristics differed between subjects who had a positive or a negative FBP-PET scan per quantitative cut-off method. The purpose was to identify clinical characteristics associated with either a positive or a negative FBP-PET scan, which could be used by clinicians to identify patients at greater risk for being clinically misdiagnosed as AD.
Methods
Subjects and Design
Baseline (pre-study drug administration) demographic, cognitive, neuropsychiatric, and FBP-PET data were obtained from a pooled subset of 199 patients (out of a total pooled population of 2,648) who enrolled in an optional imaging addendum to two multisite randomized registration trials of an investigational anti-Aβ therapy, semagacestat. The imaging addendum was available for any investigator and patient who wanted to participate if they were in an investigative site with access to FBP-PET. Both trials, IDENTITY (H6L-MC-LFAN; NCT00594568) and IDENTITY−2 (H6L-MC-LFBC; NCT00762411), had identical entry criteria and enrolled male and female patients ≥55 years old with a NINCDS-ADRDA criteria diagnosis of probable AD. Inclusion was a Mini Mental State Exam (MMSE) score between 20 and 26 (inclusive) for mild stage dementia or between 16 and 19 (inclusive) for moderate stage. Females could no longer be of child-bearing potential. Patients were excluded from the trials for stroke, unstable medical illness, substance abuse/dependence, major depression, or vascular dementia. A MRI or computerized tomography (CT) scan had to have been performed within the preceding 2 years to confirm no findings were inconsistent with a diagnosis of AD.
Protocols were reviewed and approved by institutional review boards, and all patients and caregivers provided written informed consent according to the Declaration of Helsinki. A further description of the studies can be found in the report of Carlson et al.
9Rating Scales
The following rating scales were administered to patients at baseline; that is, at the time of randomization: The Alzheimer’s Disease Assessment Scale: a cognitive subscale 14-item version (ADAS-Cog14),
10,11 which has a score range of 0–90, where higher scores indicate greater impairment; the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL),
12,13 which has a score range of 0–78, where higher scores indicate greater independence in the performance of ADLs; the Clinical Dementia Rating–Sum of Boxes (CDR-SB),
14 which has a score range on a scale of 0–18,
15 where higher scores indicate more severe impairment; the MMSE,
16 which has a score range of 0 (very impaired)–30 (normal); and the Neuropsychiatric Inventory (NPI),
17 a 12-item assessment of psychopathology in neurologically impaired patients, where scores range from 0–144. Also included were the mood, frontal, and agitation subscales in addition to a two-item psychosis measure
18–21 and the Geriatric Depression Scale (GDS).
22 The GDS has a score range from 0–15, where higher scores indicate more symptoms and a cut-off of 7 points is consistent with syndromal depression.
22 Patients had to score ≤6 to be eligible for the IDENTITY studies.
Neuroimaging
FBP-PET neuroimaging was performed on a subset of trial participants who agreed to participate in the optional addendum to these trials. Florbetapir F18 [(E)−4-(2-(6-(2-(2-(2–18F-fluoroethoxy)ethoxy)ethoxy)pyridin−3-yl)vinyl)-N-methyl benzenamine] was prepared by the method of Choi et al.
23 PET imaging was performed 50 minutes (±5 mins) after intravenous administration of 370 MBq of FBP. Images were obtained over 30 mins (6×5-mins 3D emission scans). Data for attenuation correction were collected via a transmission scan and obtained either before or after the emission scan. Following acquisition, the images were reconstructed via iterative algorithms. Quantitative analyses were performed by normalizing the summed PET frames to a standard template in Talairach space and obtaining a SUVR for a composite of cortical regions of interest (ROI) (frontal, temporal, parietal, precuneus, anterior cingulate, and posterior cingulate). The entire cerebellum was used as the reference region. Patients with a composite cortical SUVR ≥1.1 were considered to have a positive scan.
6,7Statistical Analyses
Baseline data from the subsets of patients from the IDENTITY and IDENTITY−2 trials who received FBP-PET neuroimaging were pooled for these retrospective, cross-sectional analyses. The demographic, rating scale, and cognitive data were compared by either a two-tailed Fisher’s exact test (for categorical variables) or analysis of variance (ANOVA, for continuous variables) between groups of patients categorized as positive or negative for Aβ on FBP-PET. Correlations between the composite SUVR and age or years of education were assessed using the Pearson product-moment correlation test.
Results
As summarized in
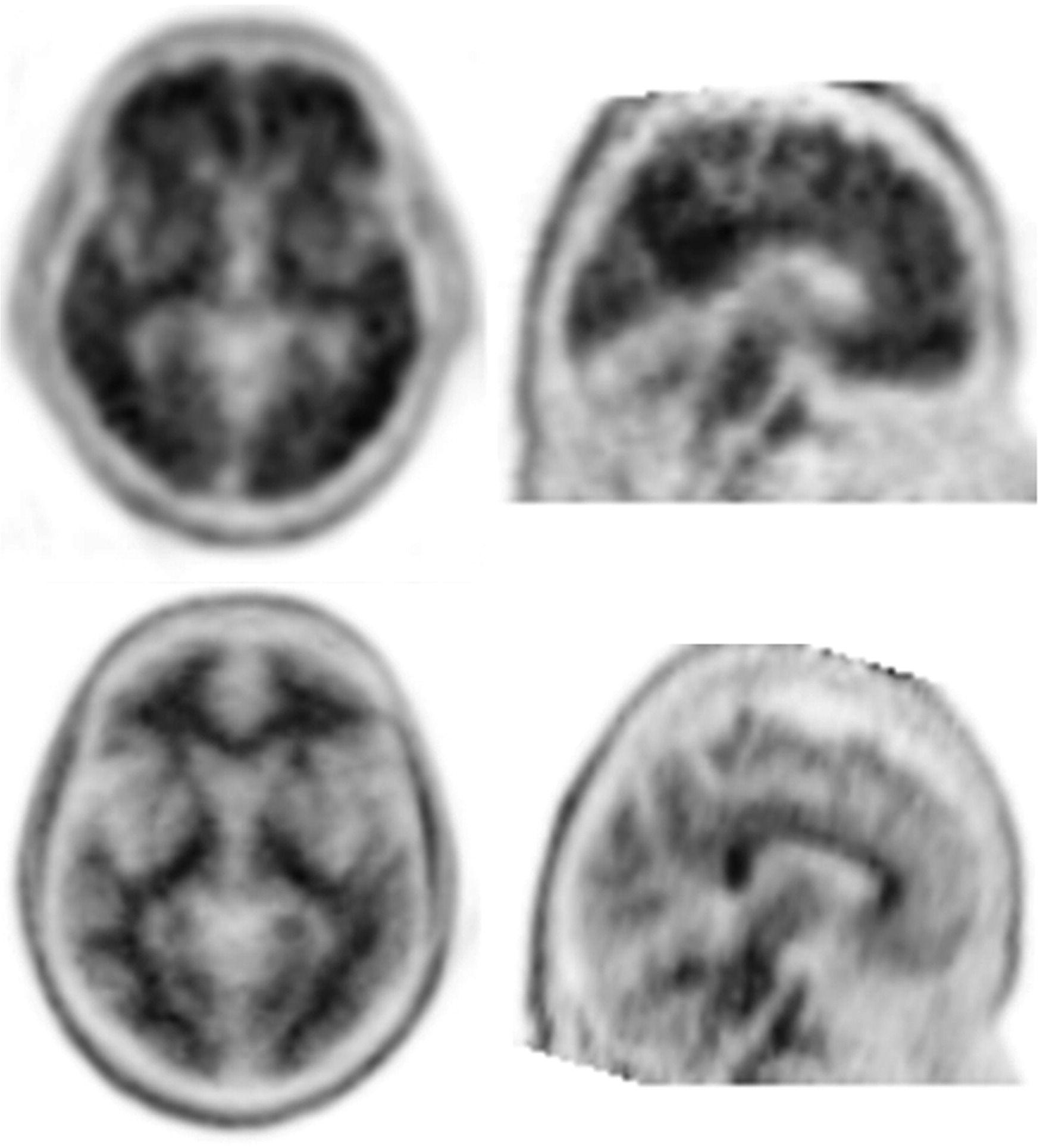
Table 1, of the 199 patients across the two trials who underwent FBP-PET imaging at baseline, 164 (82%) were categorized as positive (SUVR ≥1.1) and 35 (18%) as negative for amyloid plaque (see
Figure 1 for examples of a negative and positive scan). Negative and positive groups had a similar mean age (74.3 versus 75.5 years; p=0.449) and years of education (14.8 versus 14.9 years; p=0.809), and neither age (r=−0.002; p=0.980) nor years of education (r=−0.008; p=0.915) were found to correlate with the FBP-PET composite SUVR (data not shown). The FBP-PET negative scan group had a significantly lower proportion of females (37% versus 58%; p=0.039), were significantly more likely to have a clinical diagnosis of mild stage (MMSE 20–26) AD dementia (86% versus 59% in the negative scan and positive scan groups, respectively; p=0.003), and were significantly more likely to be noncarriers of the ApoE ε4 allele (82% versus 33% in the negative scan and positive scan groups, respectively; p<0.001). There was no significant effect of race observed (data not shown).
The FBP-PET negative scan group performed significantly better on the baseline ADAS-Cog14 and MMSE than the positive group; however, both groups scored in the abnormal range. There was no distinguishing pattern of abnormalities in the items of the MMSE. In contrast, patients in the FBP-PET negative scan group had a worse performance on the GDS than those in the positive scan group [mean (standard deviation, SD): 2.23 (1.70) versus 1.51 (1.42); p=0.010]. No difference between the FBP-PET negative and positive scan groups were found on the ADCS-ADL [mean (SD): 63.8 (11.6) versus 62.4 (11.6); p=0.527], CDR-SB [mean (SD): 4.44 (2.86) versus 5.19 (2.80) ; p=0.158], the NPI total score [mean (SD): 12.1 (16.9) versus 10.9 (12.1) ; p=0.643], or any of the three NPI subscales.
In contrast to the results of patients with a clinical diagnosis of mild AD, of whom 24% had negative FBP-PET scans, among all patients with a clinical diagnosis of moderate stage AD dementia only 7% were in the FBP-PET negative group and 93% had positive FBP-PET scans. Among all ApoE ε4 carriers, only 6% were in the FBP-PET negative group whereas 94% were in the FBP-PET positive group. Among ApoE ε4 noncarriers, 37% had negative FBP-PET scans.
Discussion
We retrospectively analyzed pooled clinical and FBP-PET scan data from the pretreatment baseline visits from two industry-sponsored clinical trials with identical entry criteria for 199 patients who had been clinically diagnosed by dementia experts using NINCDS-ADRDA criteria as having mild or moderate probable AD dementia. Overall, we found that 35 patients (18%) had a negative FBP-PET scan; that is, they lacked evidence of amyloid pathology despite having received a clinical diagnosis of AD. Negative FBP-PET scans were more common in ApoE ε4 noncarriers and patients with mild AD dementia.
Sperling and colleagues
26 reported at the 2012 Clinical Trials in Alzheimer’s Disease conference that 16% of patients enrolled in two trials of an investigational antiamyloid treatment (bapineuzumab) had negative amyloid-PET results (using
11C-PiB). In that same report, and similar to our findings, 36% of ApoE ε4 noncarriers were PiB-PET negative compared with only 7% of ApoE ε4 carriers. Similarly, Johnson et al.
27 recently reported that 16% of patients in another study who had been clinically diagnosed (NINCDS-ADRDA) with AD dementia (MMSE ≤24) were FBP-PET negative, all of whom were ApoE ε4 noncarriers. Landau et al.
25 analyzed data from the Alzheimer’s Disease Neuroimaging Initiative and reported 23% of all clinically diagnosed AD dementia patients in their study were FBP-PET negative. Additionally, Fleischer et al.
28 reported that 15% of clinically diagnosed AD dementia patients in their cohort were found to be FBP-PET negative.
The FBP-PET negative scan group showed significantly less impairment on the ADAS-Cog14 and MMSE (both p<0.001), and fewer of these patients were in the moderate AD severity group compared with those with FBP-PET positive amyloid scans. The finding of a relationship between more cognitive impairment and FBP-PET scanning is consistent with recent research showing a significant positive correlation between cognitive impairment and FBP-PET SUVR values.
24 Landau et al.
25 also reported a significant positive correlation between amyloid load using FBP-PET and cognitive impairment using the ADAS-Cog in early mild cognitive impairment (MCI) (r=0.24; p=0.002) and late MCI patients (r=0.29; p=0.007).
As would be expected, patients in our FBP-PET negative scan group were also less likely to be ApoE ε4 allele carriers, where ApoE ε4 is a well-known risk factor for amyloid and AD. Additionally, although patients with syndromal major depression at baseline were excluded from our studies, patients in the negative scan group exhibited significantly higher levels of depression symptoms than the positive scan group. However, the clinical significance of this finding is unclear because the mean baseline GDS scores for both groups were well below the cut-off for syndromal depression.
We focused on the baseline data acquired at the trials’ pretreatment visit in order to better simulate and inform clinical practice. We sought to identify variables that could be used by clinicians at this stage to identify patients at greater risk for being clinically misdiagnosed as AD. Although we detected some differences between scan positive and scan negative groups, the most striking finding was that few clinical descriptors distinguished these groups. While negative FBP-PET scans were more common in ApoE ε4 noncarriers and patients with mild AD dementia, suggesting diagnosis may be more difficult in these patients, there was a large degree of overlap in the ranges of cognitive scores and demographic characteristics between the groups (
Table 1), and none of those factors are sufficient for an accurate prediction of which demented patients diagnosed with probable AD per clinical criteria lacked Aβ neuropathology.
In addition to clinical practice, these data also support the idea that the use of biomarkers in the patient selection process can add value to AD clinical trials where investigational treatments are being studied in both dementia and MCI populations. This would enhance the detection of an efficacy signal in the trial and decrease exposure of patients who do not have AD to risks associated with investigational medicines. Further research is needed across all phases of AD to understand the relationship among various biomarkers and their place in the clinic.
A limitation of our report is that only the subset of 199 patients underwent FBP-PET neuroimaging and they may not be representative of the larger study sample; nonetheless, the proportion (18%) who were diagnosed as having AD by dementia specialists but had no amyloid biomarker evidence for AD mirrored that reported in the literature.
1,2,25,27,28 We cannot speculate as to the negative-scan patients’ true causes of dementia. However, in a different type of patient cohort, Beach et al.
2 reported a wide variety of pathological findings on autopsy for those who were misdiagnosed antemortem without the benefit of amyloid imaging, including tau-only dementia, frontotemporal lobar dementia, and cerebrovascular disease. Finally, we used a quantitative method to determine dichotomous scan status (positive or negative for amyloid). Using a qualitative read may have resulted in different findings, although there is high comparability between these methods.
6,7The value of a FBP-PET scan for a more accurate AD diagnosis is highlighted by our findings in demented patients. Our findings add to a growing literature about the challenges of making an accurate diagnosis solely on clinical phenotype even where structural neuroimaging has excluded other etiologies.
Conclusions
A certain diagnosis of AD remains challenging to the clinician and researcher without antemortem confirmation of Alzheimer’s pathology. Although diagnosed by expert clinicians, 18% of patients with probable AD dementia in this study had FBP-PET results inconsistent with a diagnosis of AD. The Aβ-positive FBP-PET scan group demonstrated significant deficits on several cognitive tests compared with patients with negative scans at baseline; however, there was a large degree of overlap between the groups and no single factor can be said to predict a negative scan. This study highlights the importance of amyloid neuroimaging in the AD diagnostic matrix. The use of FBP-PET could provide valuable assistance to clinicians in their assessment of patients presenting with cognitive deficits.
Acknowledgments
The authors report that they are employees of Eli Lilly. All authors except Dr. Quinlivan are minor shareholders with Eli Lilly.
This study was supported by Eli Lilly.
The authors thank all the patients who participated in this study and the optional imaging addendum.