Cortical thinning has been detected in clinically normal (CN) elderly individuals with greater amyloid burden seen on Pittsburgh Compound B (PiB) positron emission tomography (PET)
11,12 and apathy has been associated with increased cortical amyloid burden in MCI.
13 Accordingly, cortical thinning has been detected in CN individuals with low cerebrospinal fluid amyloid-β
1–42.
14 Therefore, atrophy associated with apathy in AD dementia patients may begin during MCI and possibly even in CN individuals at risk for AD. Although apathy is known to manifest at the stage of MCI, which can represent prodromal AD, the possibility of apathy-associated atrophy in CN individuals possibly at the preclinical stage of AD remains relatively unexplored. In a study of mild AD dementia, MCI, and CN individuals from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), Donovan et al. found that reduced baseline inferior temporal cortical thickness was predictive of greater apathy over time across all diagnoses.
15 This reduced thickness may have indicated cortical atrophy associated with AD pathology, atrophy associated with normal aging, or innately reduced inferior temporal cortical thickness which could predispose toward the development of apathy. If AD-induced, apathy-associated cortical atrophy is found in the preclinical or prodromal stages of disease, it may be a useful biomarker for clinical trials involving the earliest stages of AD. Furthermore, not all cases of MCI advance to AD dementia.
16 If detection of apathy with regionally specific atrophy in MCI or CN individuals is predictive of such progression, these markers may be useful for earlier and more accurate diagnosis of AD.
In this study, we set out to investigate the possibility of apathy-associated regionally specific cortical atrophy in MCI and CN individuals cross-sectionally. To evaluate apathy, we employed the clinician-rated Apathy Evaluation Scale (AES-C) (made up of 18 items),
17 a comprehensive method of quantifying many aspects of apathy. MRI was employed to assess regional cortical thickness. We hypothesized that greater apathy in MCI and CN individuals would be associated with atrophy of medial frontal regions, as has been demonstrated previously in AD dementia,
8,10 and inferior temporal atrophy as recently seen in individuals with milder cognitive impairment.
15 Detection of such apathy-associated cortical atrophy in MCI or CN individuals may provide opportunities for earlier AD diagnosis and enriched clinical trial designs.
Results
Demographics and characteristics for all subjects and each diagnostic group are displayed in
Table 1. Compared with CN subjects, MCI subjects demonstrated significantly lower MMSE scores, Digit Symbol scores, RAVLT total learning scores, and AES-C scores (indicating greater apathy), and significantly higher CDR sum of boxes scores. The MCI group also had a higher percentage of males than the CN group. Lower AES-C score (greater apathy) was associated with lower MMSE (r=0.29, p=0.02), Digit Symbol (r=0.34, p=0.007), and RAVLT total learning scores (r=0.39, p=0.001), and with higher CDR sum of boxes scores (r=−0.583, p<0.001).
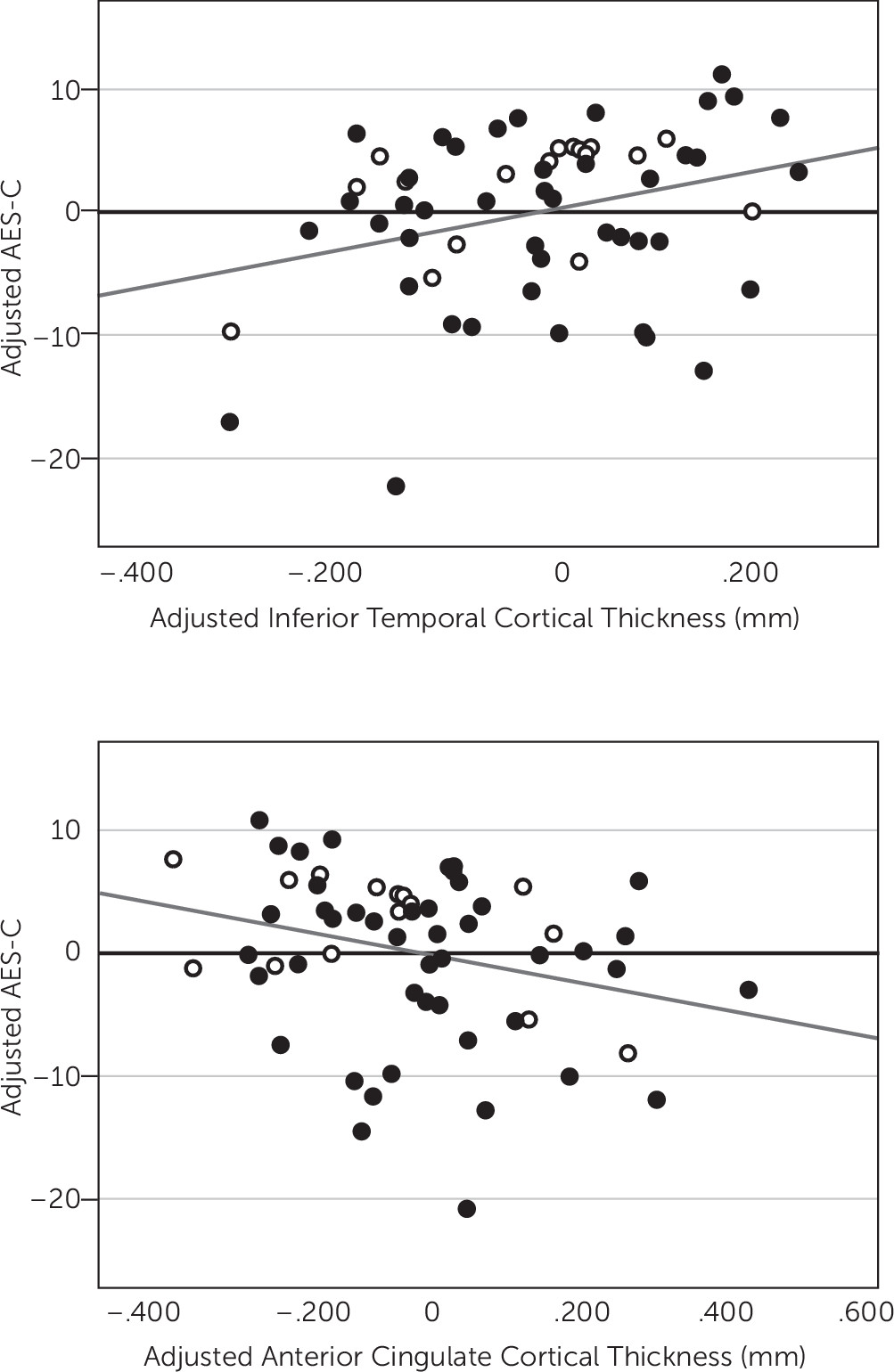
After backward elimination, the multivariate linear regression model indicated a significant association between lower AES-C scores, representing greater apathy, and lower inferior temporal cortical thickness (p=0.004) and greater anterior cingulate cortical thickness (p=0.04), see
Table 2 and
Figure 1. Thickness of other cortical ROI was not significantly associated with AES-C score. Other predictors retained in the model included diagnosis, where an MCI diagnosis was more associated with lower AES-C score (greater apathy) than a CN diagnosis (p=0.0007) (R
2=0.31, p=0.0001 for model), see
Table 2. Of note, there was a moderately strong positive unadjusted univariate correlation between inferior temporal and anterior cingulate cortical thickness (r=0.51, p<0.0001) suggesting possible multicollinearity as an explanation for the unexpected inverse relationship of AES-C score with anterior cingulate cortical thickness seen in the model. However, the unadjusted univariate correlation between anterior cingulate cortical thickness and AES-C score, which does not reach statistical significance (r=−0.12, p=0.37), was negative as it was in the model.
Discussion
In this study, we sought to identify regions of lower cortical thickness suggestive of atrophy that are associated with apathy in individuals possibly at the prodromal stage of AD, characterized clinically as MCI, and CN elderly. The results of our study suggest that lower thickness of the inferior temporal cortex is associated with greater apathy in both CN and MCI individuals after adjusting for diagnosis, age, premorbid intelligence, memory performance, and processing speed. Our results also suggest an association between greater anterior cingulate cortical thickness and greater apathy in this population.
Investigational AD treatments at the stage of mild-moderate dementia have failed to demonstrate clinical efficacy. A potential explanation for these negative results is late therapeutic intervention at a stage of the disease at which there is already advanced pathology. For clinical trials to be successful, superior biomarker profiles for earlier diagnosis and intervention may be needed. It is well established that a significant proportion of individuals with MCI is at the prodromal stage of AD,
16,28,29 and biomarkers indicative of AD pathology have already been found preceding MCI in CN elderly individuals.
11,28,30 Such promising early biomarkers include fibrillar amyloid-β as visualized by PiB PET. However, CN individuals who may or may not develop AD have also been shown to be PiB-positive; therefore, this marker may not be sufficient to predict progression to AD dementia alone.
11,30 Other biomarkers are also needed.
Apathy is well-established as the most common neuropsychiatric symptom of AD dementia and one of the most common in MCI.
1–3 It is also predictive of impaired instrumental activities of daily living in AD dementia and progression from MCI to AD dementia.
5,7,31 Apathy has been shown to correlate with regionally specific cortical changes in AD dementia, including microstructural alterations in white matter anisotropy of the left anterior cingulate cortex,
20 reduced metabolism and perfusion of the anterior cingulate cortex
21–25 and the orbitofrontal cortex,
21–23,25 decreased perfusion of the right temporoparietal cortex,
32 and decreased perfusion of the prefrontal cortex and anterior temporal cortex.
33 Apathy has also been associated with greater neurofibrillary tangle burden of the anterior cingulate cortex
34 in moderate to severe AD dementia and increased cerebrospinal fluid total tau and phospho-tau in mild AD dementia.
35Multiple studies have demonstrated an association between apathy and atrophy of the medial frontal regions in AD dementia. While Apostolova et al. found reduced gray matter thickness of the bilateral anterior cingulate and left medial frontal cortex (supplementary motor area),
8 Bruen et al. found cortical thinning of the anterior cingulate cortex bilaterally, frontal cortex bilaterally, head of the left caudate nucleus, and bilateral putamen.
9 Tunnard et al. partially corroborated both studies with findings of atrophy in the left caudal anterior cingulate cortex, left lateral orbitofrontal cortex, and left superior and ventrolateral frontal regions.
10 Associations between apathy and atrophy of the medial frontal regions have also been found in related neurodegenerative dementias.
36 Moreover, associations between apathy and cortical amyloid burden have been detected as early as MCI.
13 Since cortical thinning patterns associated with AD have been detected as early as the preclinical stages of AD in PiB-positive CN individuals,
11,37 it is plausible to hypothesize that apathy-associated patterns of atrophy may begin at the stage of MCI or earlier.
Our findings regarding lower inferior temporal cortical thickness build on previous studies suggesting a role for lower inferior temporal cortical thickness as a predictor of greater apathy over time and the detection of inferior temporal cortex atrophy in the early stages of MCI.
15,27 In a study using ADNI data, Donovan et al. found reduced baseline inferior temporal cortical thickness to be predictive of increasing apathy over time across the AD continuum, including CN, MCI, and mild AD dementia subjects.
15 Unlike the current study, this study did not detect a significant cross-sectional association between decreased thickness of the inferior temporal cortex and greater apathy; however, this may be due to the scale used to measure apathy, the Neuropsychiatric Inventory brief questionnaire (NPI-Q), which only features a single item for apathy with a possible score range of 0–3.
38 Many other clinical-imaging correlation studies of apathy in AD dementia have used the full NPI scale, which has a wider range of scores (0–12),
39 but often was split into a dichotomous variable (present or absent apathy).
8–10,25,33 More comprehensive and sensitive measures of apathy, such as the AES employed here (possible score range 18–72), may reveal subtler cross-sectional associations between cortical thickness and apathy in individuals who may be in the early stages of AD. In a separate study using ADNI data, McDonald et al. identified accelerated inferior lateral temporal atrophy starting in the early stages of MCI (CDR sum of boxes 0.5–1.0) and continuing into mild AD dementia.
27 Coupled with our findings, these studies suggest that inferior temporal atrophy may occur in individuals in the prodromal stages of AD and that regional atrophy in these stages of disease may be associated with the presence of apathy.
However, our results are cross-sectional rather than longitudinal. Therefore, it is unclear whether the lower inferior temporal cortical thickness associated with apathy is the result of atrophy over time or an innate life-long trait which predisposes to apathy. Furthermore, while most of the subjects in this study are at high risk for developing AD dementia, we did not know which subjects or how many ultimately progressed to AD dementia. Therefore, the association between lower inferior cortical thickness and apathy may or may not be driven by underlying AD pathology. Further studies with PiB PET and longitudinal data are needed.
Despite significant evidence for apathy-associated atrophy of the anterior cingulate cortex in AD dementia,
8–10 the lack of an association between lower anterior cingulate cortical thickness and apathy in our subjects may not be entirely surprising. While McDonald et al.’s study of longitudinal cortical atrophy in MCI and AD dementia did detect inferior lateral temporal atrophy in the early stages of MCI, it did not detect atrophy of the anterior cingulate cortex until impairment increased to a CDR sum of boxes score of 1.5–2.5.
27 The mean CDR sum of boxes score in MCI subjects in the current study was 1.7 and CN subjects were included as well, potentially reducing the possibility of observing a regional association with anterior cingulate cortex atrophy and apathy. Therefore, greater apathy may be associated with anterior cingulate atrophy only in later stages of the disease than examined by the current study, while inferior temporal atrophy may be associated with greater apathy at earlier stages of AD.
It is difficult to explain the unexpected association between greater apathy and greater anterior cingulate cortical thickness observed in our study. When looking at the univariate unadjusted relationship between apathy and anterior cingulate cortical thickness, the directionality is the same as that found in the multivariate model. This suggests that the weak yet statistically significant relationship between greater anterior cingulate cortical thickness and greater apathy may be a real finding. This finding may suggest a compensatory or inflammatory response to AD pathology in the anterior cingulate cortex which precedes atrophy. Analogous phenomena have been identified in other brain regions. Functional MRI studies have shown a potential compensatory effect at the stage of early MCI with increased hippocampal activity during a memory task, which later reverses to decreased activity at the stage of late MCI.
40 The findings of increased anterior cingulate cortical thickness in CN and early MCI subjects and decreased anterior cingulate cortical thickness in mild-moderate AD dementia could be due to a similar compensatory phenomenon. However, this finding is in cortical thickness rather than function. There is some evidence to support a similar phenomenon with cortical thickness measurements in the earliest stages of AD. In an MRI study of early-onset familial AD mutation carriers, cortical thickness of the posterior cingulate, precuneus and parietotemporal association areas were greater in mutation carriers prior to the onset of cognitive impairment than in healthy controls, while thickness of the same areas after symptom onset was significantly less than that found in healthy controls.
41 In a separate study, nondemented apolipoprotein E ε4 carriers had greater cortical thickness than noncarriers, but a stronger association between aging and cortical thinning between the ages of 48 and 75 years in cortical areas known to degenerate in AD.
42The current study had several limitations. First, we were limited to observing anatomical features in the brain. Apathy may also correlate with functional, metabolic, or molecular changes in the brains of MCI and CN individuals. Techniques such as resting-state functional MRI, 18F-fluorodeoxyglucose PET, and PiB-PET may be used to investigate such possibilities in future studies. Of note, we recently reported results of a cross-sectional association between apathy and PiB burden but not FDG metabolism in a small sample of MCI subjects.
13 These findings need to be extended to CN subjects and have a longitudinal component as well. Furthermore, larger samples are needed to assess the relationship between apathy and regional cortical thickness within PiB-positive individuals who are more likely to have preclinical and prodromal AD than the inclusive group of CN and MCI subjects used in the current study, who could have been PiB-positive or negative. Because of a small sample size, we were limited in the number of ROI that could be chosen for our analyses, and decreased thickness of other cortical regions associated with apathy in early AD may have been missed. Future studies will employ whole-brain exploratory analyses with voxel-based morphometry to test for such regions. Small sample size also limited the number of covariates we could control for in our general linear model. Future larger studies may be able to adjust for additional relevant covariates and also have greater statistical power to detect subtler effects of individual predictors. The cross-sectional nature of this study was another limitation. Our study suggests that decreased inferior temporal cortical thickness is associated with greater apathy cross-sectionally. However, given the lack of longitudinal analysis, it is impossible to determine whether or not the decreased cortical thickness observed here is actually the result of pathology related atrophy or instead inherent to the subjects at baseline, predisposing them to apathy their entire life. Future longitudinal studies with both repeated MRI and clinical assessments will allow for better exploration of this question and will also help determine whether certain regional atrophy patterns predict greater apathy over time and progression of AD.