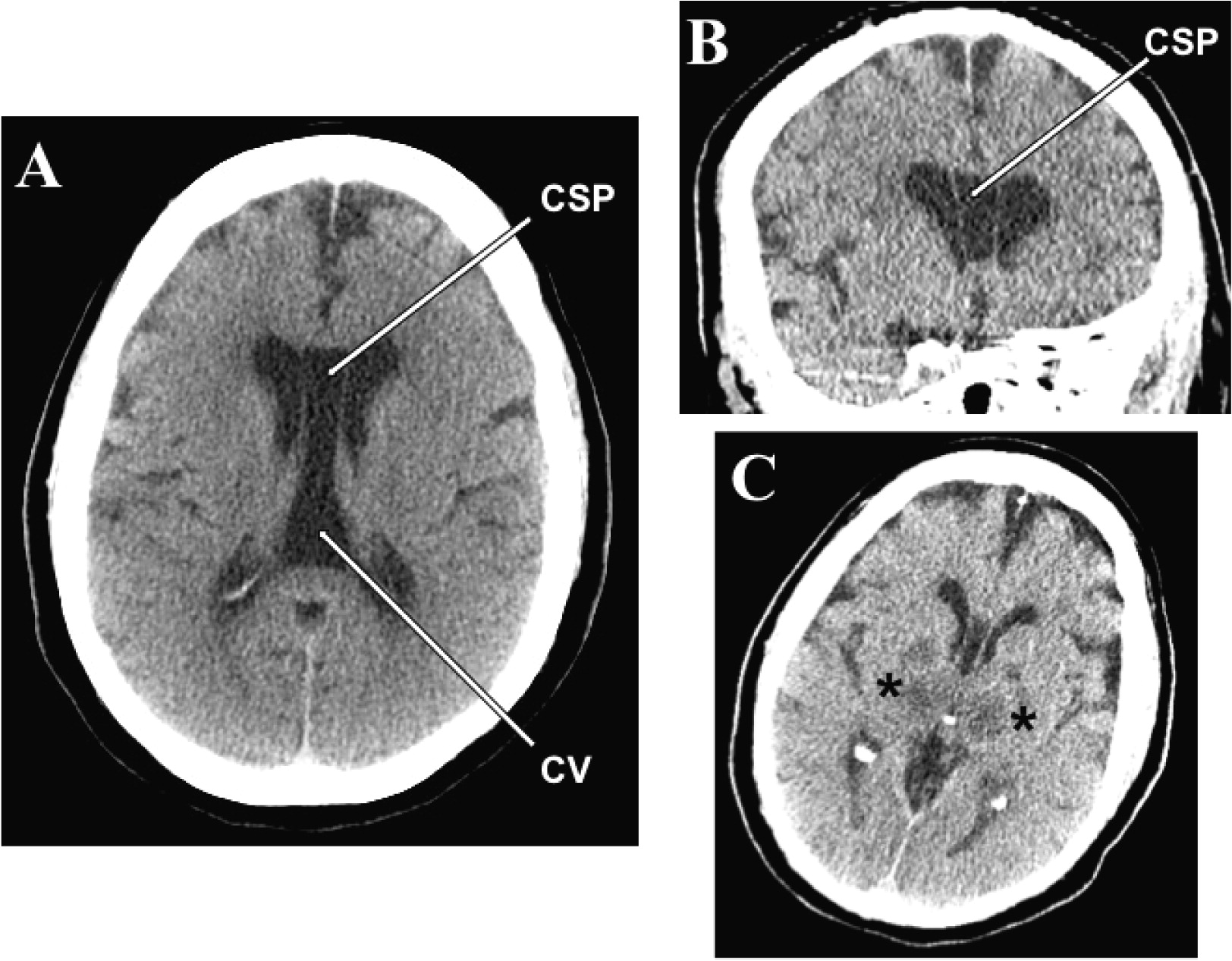
To the Editor: A case of a cavum septi pellucidi (CSP) and cavum vergae (CV) discovered incidentally in a 65-year-old woman with history of bipolar disorder with suicidal ideations is reported. The decedent died as a result of acute anoxic-ischemic encephalopathy of unknown etiology (patient was found unresponsive on the street). A head CT scan performed on admission to the emergency room showed a cystic space within the septum pellucidum with significant caudal extension consistent with a CSP/CV (
Figure 1A). The two cava were not separated (
Figure 1A). The antero-posterior length measured on CT was 5.7 cm. A follow-up head CT performed 2 days after admission demonstrated, besides the cava (
Figures 1B and 1C), hypodensities in the thalamic and lentiform nuclei bilaterally consistent with hypoxic/ischemic injury (
Figure 1C). Neuropathological evaluation at autopsy revealed a large CSP (
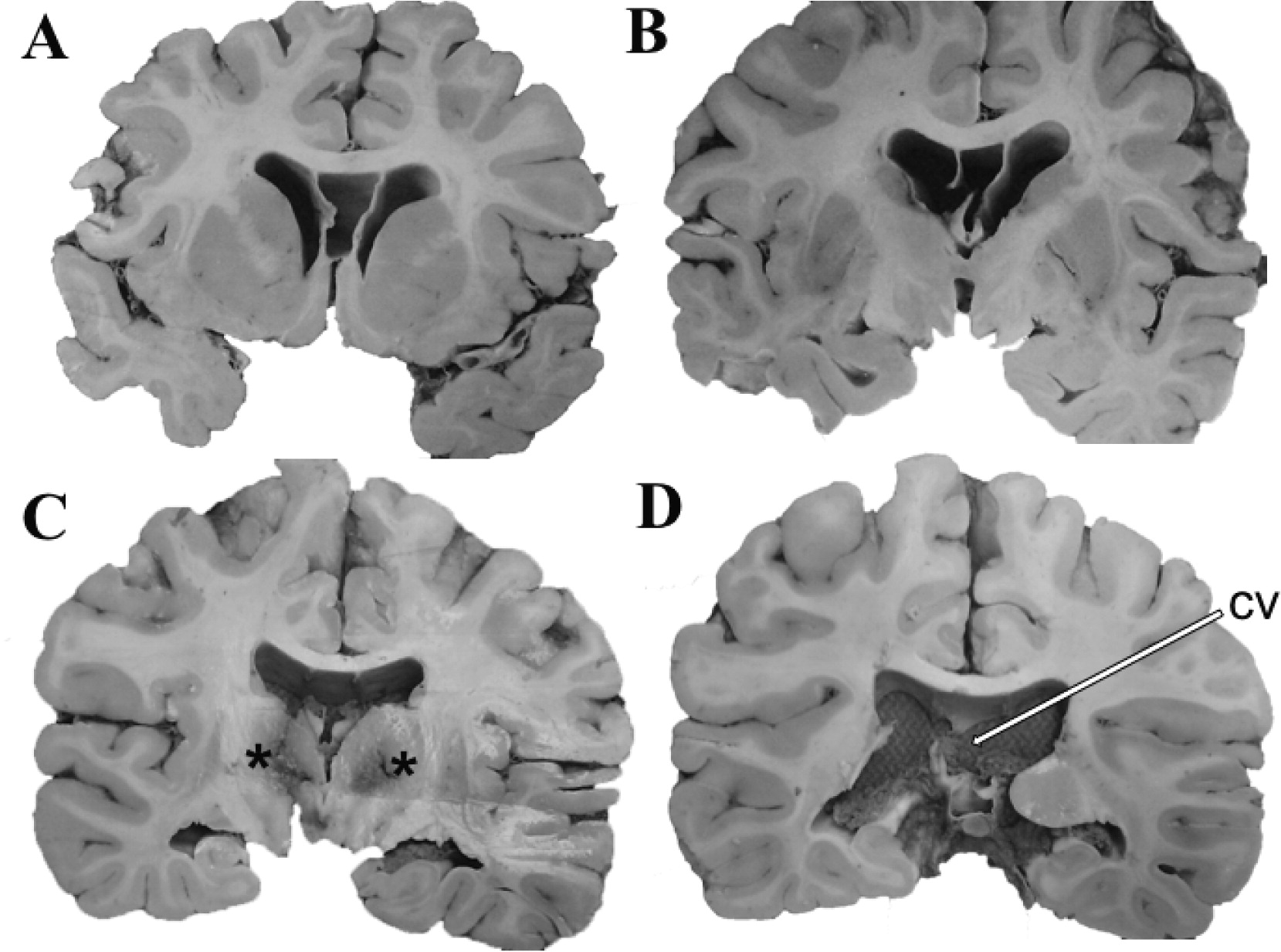
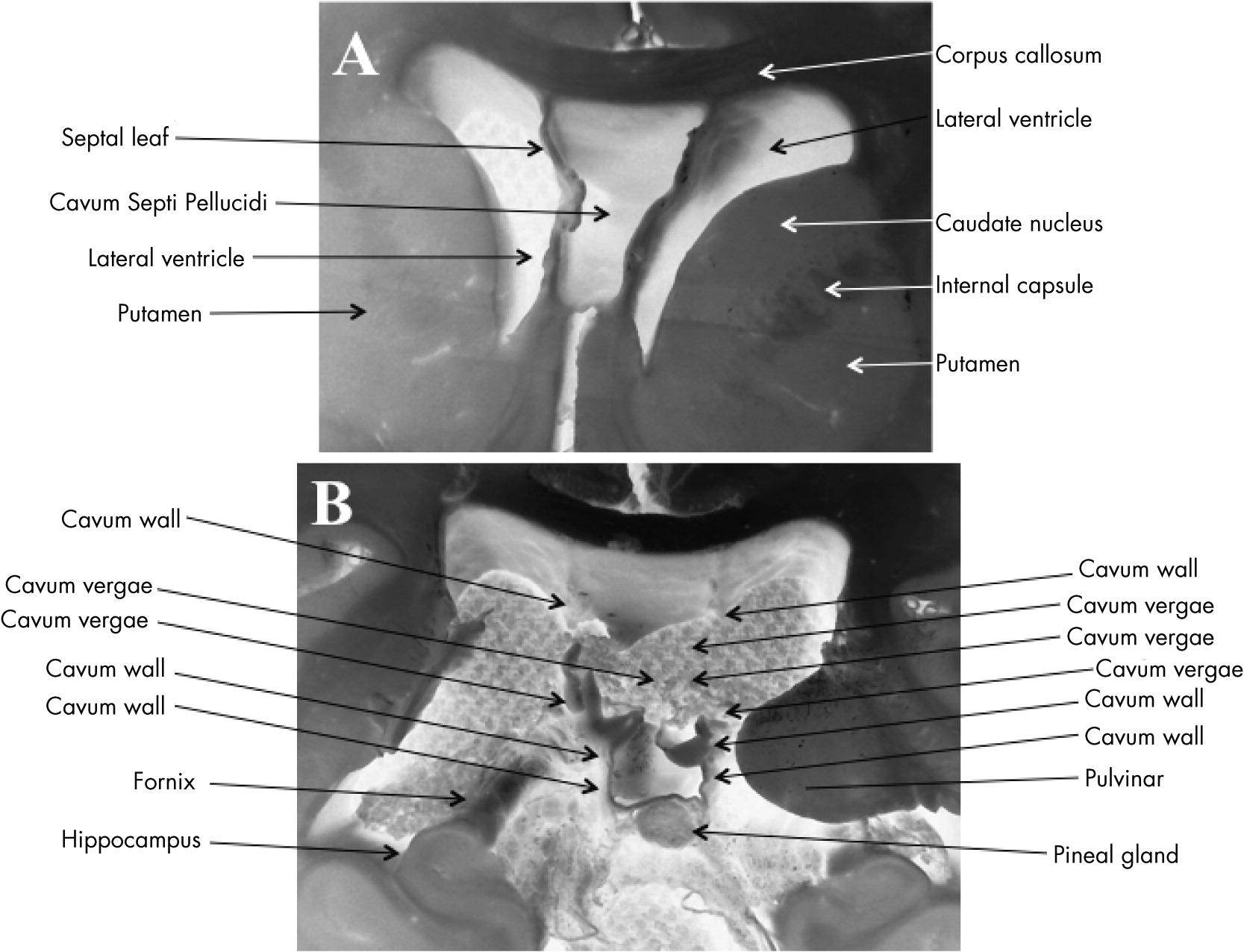
Figures 2A-
2C and
Figures 3A) extending caudally to become CSP/CV (
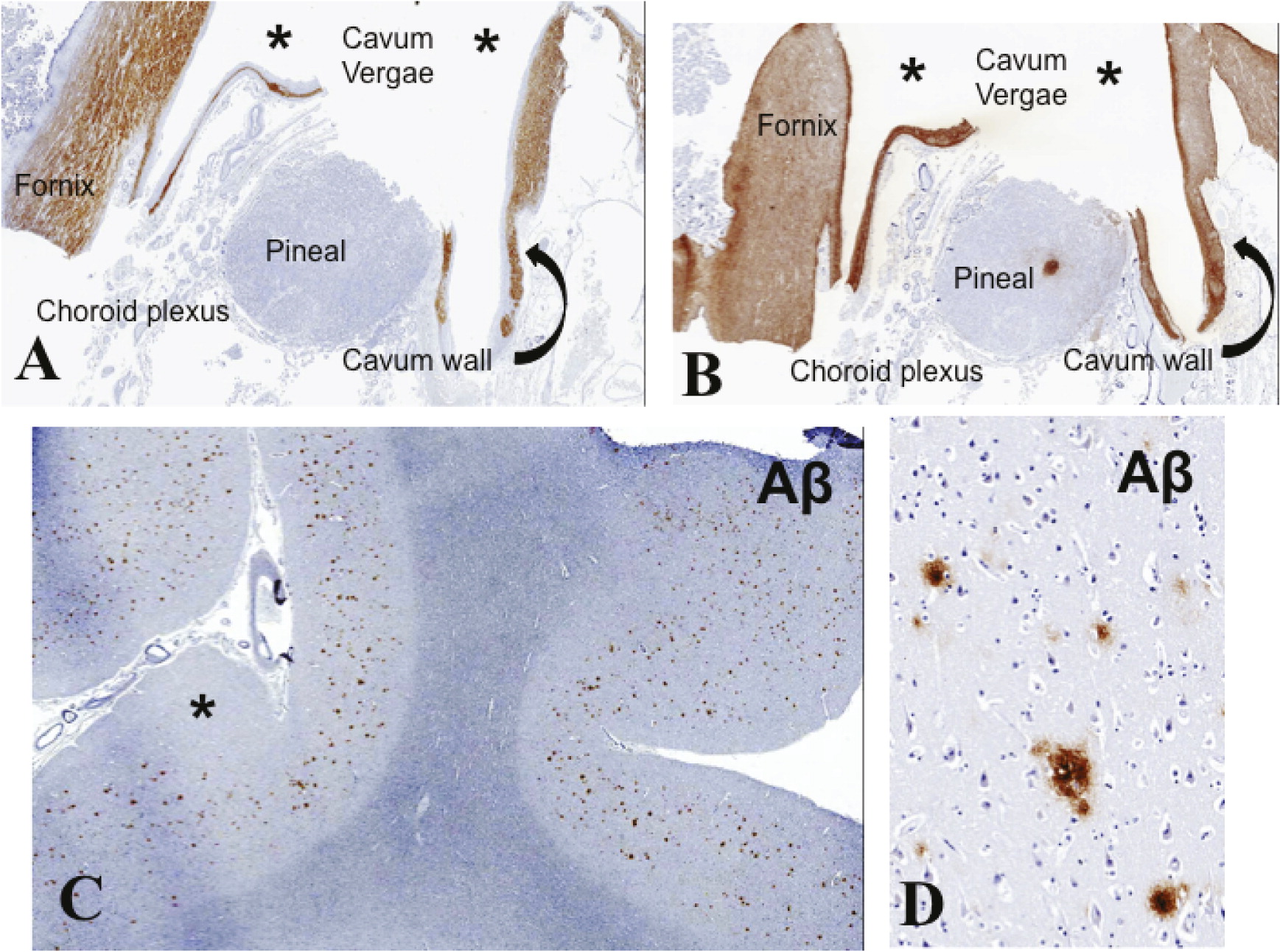
Figures 2D,
3B,
4A, and 4B). There was moderate dilatation of the lateral ventricles but no evidence of fenestrations in the septal leaves, pointing against a communication between the septal cavum and the lateral ventricles (
Figure 3A).
Diffuse hypoxic/ischemic changes were demonstrated in the hippocampus, amygdala, thalamic nuclei, and cerebral cortex consistent with cardiac arrest encephalopathy (
Figures 1C and
2C). The cerebral cortex, including prefrontal cortex and hippocampus, was developmentally unremarkable. Diffuse amyloid beta (Aβ) deposits of moderate density without neuritic plaques were noted in the transentorhinal region, entorhinal cortex, cingulate cortex, and cerebral neocortex (
Figures 4C and 4D). Neurofibrillary pathology was absent (lack of phosphorylated tau protein and α-synuclein immunoreactivity). The cerebral hemispheric white matter was essentially unremarkable.
The demonstration of CSP/CV in the present case may be significant given the decedent’s history of bipolar disorder with major depression, characterized by suicidal tendency. Although an association between CSP and psychosis, especially schizophrenia, is well established,
1–3 the relationship between CSP/CV and affective disorders is less well known.
4–7 Previous MRI studies have shown that bipolar disorder patients exhibit higher prevalence of abnormal CSP enlargement (≥6 mm in length) compared with healthy controls.
6,7 The septum pellucidum, one of the substrates of “affect” in the Papez circuit,
8 is an important relay station of the limbic system, transmitting visceral information through the hypothalamus to the hippocampus, amygdala, habenula, and the reticular formation of the brainstem, thus, regulating the emotional response to the environment.
9 A disruption of this circuit may be further complicated by the anatomical proximity of the septal leaves to the fornix, the latter originating in the subiculum, alveus, and stratum pyramidale of the hippocampus.
3 In the context of affective disorders, the functional implication of disruption of the septal connections to the anterior (subcallosal) cingulate cortex (prefrontal cortex) deserves consideration; because the septum pellucidum has a role in motivational behavior,
10 an anatomical-functional disruption of this structure may contribute to the lack of motivation commonly observed in major depressive disorder. Interestingly, CSP has also been reported in catatonia.
11 Catatonic behaviors may be encountered in major depression.
12To our knowledge, the occurrence of an increased Aβ cortical load has not been hitherto reported in the context of CSP/CV. Although it is possible that the two findings are merely coincidental, the relationship between cortical degeneration with resultant incipient brain atrophy, expanding CSP/CV, and worsening of psychotic manifestations is a subject of tempting speculation, as previously suggested.
13 The present case provides additional neuropathological evidence for a link of CSP/CV and affective disorders.