In this work, we hypothesized that at least a subgroup of these patients may also have autoreactivity against neuronal antigens.
We aimed in this study to determine the prevalence of antineuronal antibodies in Tunisian psychiatric inpatients.
Subjects and Methods
Subjects
We enrolled in this prospective study 103 psychiatric inpatients admitted to psychiatric ward F in Al Razi Mental Health Hospital (Manouba, Tunisia) during a 3-month period. All patients underwent a systematic clinical examination and an extensive neurological investigation. Forty-one healthy, age- and sex-matched blood donors were used as a control group. All control subjects underwent a careful clinical assessment investigating somatic and psychiatric morbidity. Patients and control subjects had no history of malignancies or neurological disorders. Psychiatric diagnosis was made by two different psychiatrists according to DSM-IV criteria.
10 We distinguished four groups of patients: patients with SKZ, schizoaffective disorder, and bipolar disorder and a group of the rest of the patients presenting with other psychiatric diagnoses.
Considering elementary symptoms, we also studied patients according to the presence of psychotic and/or affective symptoms as listed in the diagnosis criteria of DSM-IV.
Patients and control subjects gave their informed consent to participate in the study, and the protocol was approved by the ethical committee of Al Razi hospital.
Methods
Antineuronal antibodies were assessed by indirect immunofluorescence (IIF) using a commercially available kit (Euroimmun, Lübeck, Germany) following the manufacturer’s instructions. Briefly, sera at 1/100 were incubated on cerebellum of monkey tissue sections. Fluorescein-labeled antihuman immunoglobulin G conjugate was used as a secondary antibody. Slides were examined on a fluorescence photomicroscopeon. The ANNA-positive sera labeled the nuclei of all cerebellum cells, whereas PCA1-positive sera labeled the cytoplasm of Purkinje cells.
Subjects’ sera were also tested for antinuclear antibodies on Hep2 cells by IIF (Biomedical Diagnostics, Marne-la-Vallée, France). Positivity for these antibodies was taken into account when analyzing antineuronal antibodies.
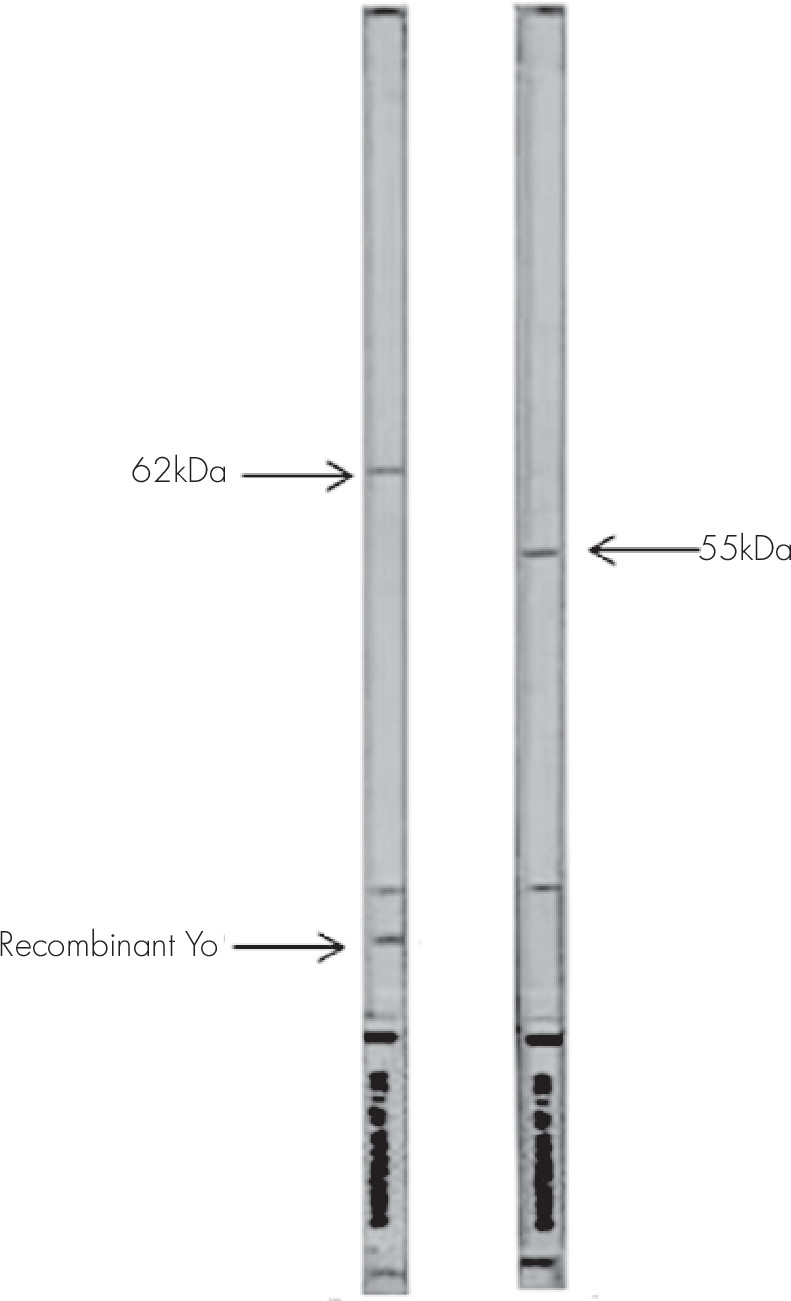
To further analyze target antigens of antineuronal antibodies, positive sera were tested with an immunoenzymatic test, combining Western blot on primate cerebellum protein extract and dot technique with highly purified recombinant Hu, Ri, and Yo neuronal antigens (Euroimmun, Lübeck, Germany). After blot strip blocking, sera were incubated at 1/51 for 1 hour at room temperature. To detect the bound antibodies, a second incubation was carried out using an alkaline phosphatase-labeled antihuman IgG. According to the manufacturer instructions, serum is considered as anti-Hu positive if it stains a band of 38 kDa and recombinant Hu simultaneously; anti-Ri positive if it stains two bands of 55 and 80 kDa and recombinant Ri simultaneously; and anti-Yo positive if it stains a band of 62 kDa and recombinant Yo simultaneously. In some cases, sera also reacted with a band of 34 kDa.
Statistical analysis was performed using SPSS 13.0 (IBM, Armonk, N.Y.). A chi-square test, analysis of variance, Dunnett's test, or Kurskal-Wallis test was used where appropriate to compare the frequency of positive antibodies between the four diagnostic groups and the control subjects and to analyze the different clinical variables across the sample. The statistical significance was based on p≤0.05.
Discussion
To date, studies on antineuronal antibodies have focused on paraneoplastic neurological disorders or other neurological conditions. The presence of antineuronal antibodies in adult psychiatric diseases has not yet been assessed.
In the present work, we studied a large cohort of psychiatric patients and found that ANNA revealed by IIF is significantly more prevalent in patients than control subjects, especially in those with SKZ and bipolar disorder. Moreover, PCA1 was associated with affective symptoms. Although first described as markers of paraneoplastic syndrome, ANNA was also found in patients with neurological syndromes of unknown etiology and occasionally in healthy individuals.
11 The IIF staining on cerebellar cryosections shows binding to neuronal nuclei, especially of Purkinje cells. Nonneuronal nuclei are not stained.
9 However, interpretation is difficult in patients with positive antinuclear antibodies, which was the case in five of our patients. None of our patients with ANNA staining had confirmed anti-Hu antibody because no reactivity was shown with recombinant Hu; however, two sera strongly reacted with 40-kDa neuronal proteins. Different kinds of atypical reactivity were already reported in sera of patients with neurological disorder without paraneoplastic syndrome. In fact, Vianello et al tested 719 sera from patients with neurological disorder and no suspicion of paraneoplastic disease by immunohistochemistry on rat cerebellum and by immunoblot of human extract cerebellum. Nine patients had anti-Hu anibodies and seven others had atypical reactivity.
12Otherwise, two of our patients with SKZ with ANNA staining had confirmed anti-Ri antibodies. It is difficult to distinguish anti-Hu and anti-Ri antibodies by IIF on primate cerebellum because the two react with nuclei of central neurons. In fact, anti-Hu antibodies react with nuclei of both central and peripheral neurons, whereas anti-Ri reacts only with the nuclei of central neurons.
9 However, it is imperative to confirm the specificity with Western blot of cerebellum extract and including recombinant antigens, which was the case in our study.
Anti-Ri antibodies are reported as rarer than anti-Hu antibodies in association with paraneoplastic syndromes and especially in gynecological and lung cancers.
13 To the best of our knowledge, these antibodies were not associated with SKZ.
It is interesting to note that one of our sera with positive ANNA by IIF was considered anti-Yo positive because it reacted simultaneously with a 62-kDa protein and recombinant Yo. This serum was also positive for antinuclear antibodies, raising the question of the difficulty in interpreting IIF when there are different types of reactivity. For this reason, the majority of authors recommend the use of Western blot analysis with recombinant antigens.
Among the seven patients and the two controls with suspected PCA1 by IIF, only two patients reacted with recombinant Yo and were considered confirmed anti-Yo antibodies.
These antibodies are also associated with paraneoplastic syndromes and especially with gynecological cancers
14; thus, they are more frequently detected in female patients, contrary to our study.
Anti-Tr antibody is another antineuronal antibody staining the cytoplasm of Purkinje cells, which could cause confusion in the interpretation of patterns. This antibody was also reported in association with Hodgkin’s disease in younger patients.
15,16 Surprisingly, anti-PCA1 antibodies were associated with younger age in patients but not in control subjects (p=0.045).
Anti-Tr antibodies cannot be confirmed by immunoblot or by using recombinant antigens because they seem recognize conformational epitopes.
Surprisingly, no association was found between the treatment and the presence of the different autoantibodies. It’s well known that neuroleptics and especially phenothiazines had a stimulating effect on B lymphocytes-producing antibodies.
17 This may be explained by the reduced number of our patients.
It has been reported that neurological symptoms of paraneoplastic syndromes could appear several years before tumor diagnosis.
11 These antibodies may predict cancers but they are not necessarily markers of an underlying tumor. In fact, after a follow-up of 5 years, none of our patients developed a neoplasm or a neurological paraneoplastic syndrome. This has already been reported in some patients with neurological manifestations
11 but never in those with psychiatric diseases.
Although they were first described as markers of paraneoplastic syndrome, antineuronal antibodies were also described in several other conditions. Most of these studies reported atypical relativities. In fact, Ben Yahiya et al
8 tested 71 patients with Gougerot Sjogren syndrome and 102 patients with lupus erythematosus and reported a high frequency of antineuronal, antibodies but only two cases had well-characterized anti-Hu antibodies.
Concerning psychiatric diseases, the presence of antineuronal antibodies has already been reported in children with obsessive-compulsive disorder and Tourette syndrome,
18,19 in the context of the controversial concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. However, in these conditions, autoantibodies detected triggered brain and basal ganglia antigens.
More recently, these antibodies were detected in autistic children. Even more, they were associated with the severity of the disease.
20,21To our knowledge, our study is the first one assessing the prevalence of antineuronal antibodies in adult psychiatric patients. We report here a high prevalence of these antibodies, especially in patients with SKZ and bipolar disorder. This finding suggests that two apparently distinct psychiatric disorders may share common etiopathogenic factors. In fact, some subgroups among these disorders may share common biological processes that participate in the pathogenesis of the clinical symptoms. This hypothesis fits with the genetic model of vulnerability in psychiatry as it is now clear that clinical diagnosis as distinct as SKZ, bipolar disorder, schizoaffective disorder, and major depression share some susceptibility genetic factors.
22,23Conversely, some authors
24,25 have proposed considering patients according to categories of symptoms and not to the classical clinical diagnosis as defined by DSM criteria. The association of PCA1 to the presence of affective symptoms in patients with different diagnosis supports the latter hypothesis.
Thus, in our patients where an antigen–antibody reaction was found triggering classical or unknown targets, this feature may suggest an immunological basis for disease pathogenesis and may be responsible for a range of symptoms. However, these antibodies may also be produced as a consequence of a local damage or by alternative immune mechanisms.