Disinhibition, the inability to inhibit inappropriate behavior, involves impulsivity, poor risk assessments, and disregard for social conventions.
1 Disinhibition interferes with the ability to inhibit automatic behaviors, urges, and emotions and impedes goal-directed behavior, such as resisting temptation, delaying gratification, and controlling impulses,
2–4 and therefore can lead to reduced social acceptance. It is not known whether the same mechanisms are responsible for both social inhibition and lower levels of inhibitory control. Dimitrov et al.
5 stated that social inhibition may be at the top of the control versus automatic continuum, whereas inhibition of simple movement may be at the bottom. In this paper, we focused on social inhibition rather than motor responses.
Evidence indicates that the right inferior frontal cortex is important in behavioral inhibition, including cognitive processes, social behavior, and inhibition of motor responses. Damage to the right inferior frontal cortex lowers performance in executive control tasks, most likely by disrupting inhibition.
6 The left prefrontal cortex and anterior cingulate cortex are also involved in inhibition. The left prefrontal cortex may help prepare upcoming behavior, maintain an appropriate task set, and correct behavior following an error.
7,8 The anterior cingulate cortex is involved in conflict detection and evaluative processes indicating when control needs to be more strongly engaged.
8 A voxel-based morphometry study of patients with Alzheimer’s disease found that greater behavioral disinhibition, as measured using the Neuropsychiatric Inventory (NPI),
9 a structured, caregiver-based interview, was strongly associated with reduced gray matter volume in the right middle frontal and precentral gyri and bilateral cingulate.
10We used the NPI to record observations of behavioral disinhibition in a large sample of patients with penetrating traumatic brain injury (pTBI). As lesion studies provide complementary information to neuroimaging studies, we performed a whole-brain voxel-based lesion-symptom mapping analysis (examining voxel-by-voxel the relationship between NPI disinhibition scores and lesion locations) to investigate the brain regions responsible for behavioral inhibition. We predicted that areas in the brain associated with this measure would include frontal regions in the right hemisphere, the anterior cingulate cortex, and precentral gyri.
Discussion
We used a whole brain approach to investigate specific brain regions responsible for behavioral disinhibition in a large sample of patients with pTBIs. Nineteen percent of our patients with pTBIs were rated by their caregiver as exhibiting behavioral disinhibition. This rate is similar to that for patients with Alzheimer’s disease,
10,31 lower than the rate for a younger group of patients with severe diffuse axonal or focal TBI,
32 and higher than the rate for patients with Parkinson’s disease
33 or corticobasal degeneration.
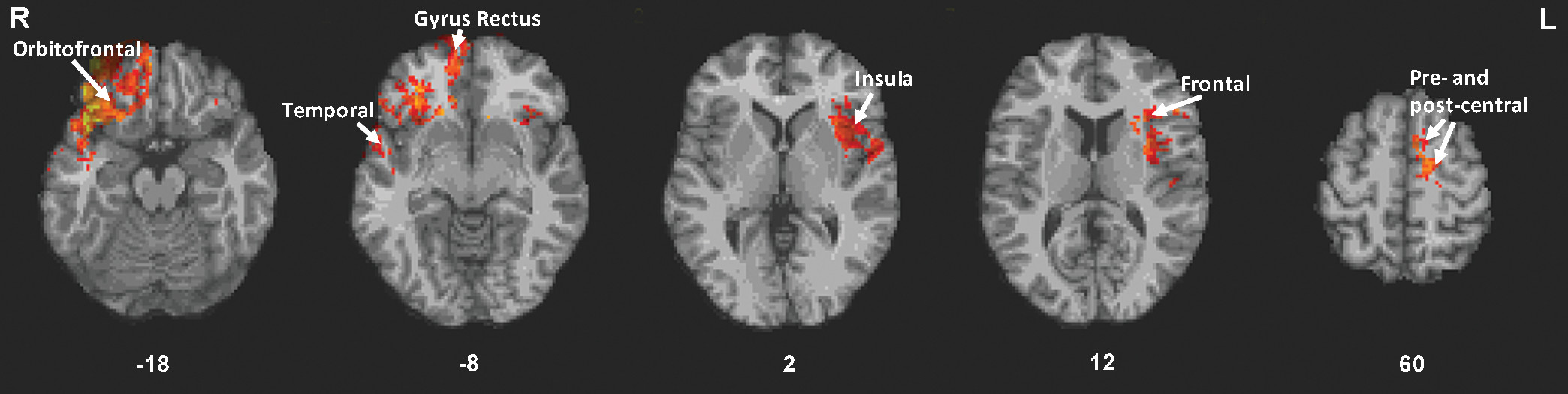
34Our voxel-based lesion-symptom mapping analysis revealed an association between greater behavioral disinhibition and the following regions: mainly right orbitofrontal regions, bilateral insula, right temporal lobe, left frontal, precentral and postcentral regions, and bilateral gyrus rectus. Other patient studies showed similar findings. For example, patients with ventral frontal lesions had greater disinhibition than patients with nonventral frontal lesions.
35 In patients with frontotemporal dementia, a positive correlation between NPI disinhibition and glucose hypometabolism in the bilateral ventromedial orbitofrontal cortex/gyrus rectus, was found.
36 Similarly, in individuals with mild cognitive impairment or dementia, a positive association between NPI disinhibition and bilateral orbitofrontal cortex atrophy was revealed.
37 In a voxel-based morphometry study of patients with frontotemporal dementia, NPI disinhibition was associated with atrophy in regions similar to those we found, including the bilateral orbitofrontal cortex, bilateral inferior frontal cortex, bilateral insula, and right middle temporal regions.
38 Another study of patients with behavioral variant frontotemporal dementia and Alzheimer’s disease found that NPI disinhibition was associated with atrophy in the bilateral orbitofrontal cortex and left temporal pole.
39 The same study found that atrophy in the bilateral orbitofrontal cortex, subgenual areas, medial prefrontal cortex, and temporal pole covaried with errors in inhibiting prepotent verbal responses.
Our results are consistent with the involvement of the right orbitofrontal cortex and inferior frontal cortex in disinhibition, as demonstrated by the above studies, and support this area’s importance in self-control
3 and response stopping.
40 They add to evidence that the ventral frontal cortex is crucial not only in motor response inhibition but also in the control of social behavior. Dillon and Pizzagalli
41 suggested that the right ventrolateral prefrontal cortex may support a general inhibitory process. In addition to the right orbitofrontal cortex/inferior frontal cortex, we found frontal regions in the left hemisphere, including inferior, middle, and superior frontal areas, that when damaged were associated with increased disinhibition. This is consistent with the left inferior, middle, and superior frontal cortex activation found in the incongruent versus neutral condition in a Flanker task,
42 although those authors also found similar regions on the right. Aron et al.
6 suggested a functional division in inhibition where the left prefrontal cortex’s role is maintaining goals/sets, the anterior cingulate cortex’s is to detect conflict when the current condition does not match those goals, and the right inferior frontal cortex’s is to suppress irrelevant responses.
The bilateral gyrus rectus involvement that we found is consistent with a study showing that patients with gyrus rectus lesions performed poorly on the Trail Making B test, which is considered an inhibition task.
43In addition to frontal areas, we found lesioned areas in the bilateral insula associated with disinhibition. Insula activation has been found during a Flanker task
42 and a response inhibition task.
7 The insular cortex has been implicated in task-set maintenance and top-down control.
44 We also found that damage to the right temporal lobe, including the pole, was associated with disinhibition. Consistent with this, patients with temporal variant frontotemporal dementia with atrophy in either temporal lobe exhibited disinhibition.
45 In addition, disinhibition, as measured by the Frontal Systems Behavior Scale, was associated with gray matter loss in the right medial and superior temporal lobe in patients with frontotemporal dementia.
46 The temporal lobe is also involved in inhibiting prepotent verbal responses.
39Motor inhibition tasks often activate parietal areas, but we did not find an association between parietal damage and disinhibition. We had fewer patients with damage to parietal regions and therefore had less power to detect an association there; however, inferior parietal lobe activation during response inhibition has been shown to be stronger in women,
47 whereas our sample included only men. In addition, no impairment was found in Stop-Signal task performance during disruptive transcranial magnetic stimulation over the right parietal region,
48 suggesting that it may not be crucial for inhibition. Alternatively, long-range connectivity between the frontal and parietal regions may have been disrupted in our patients with TBI, degrading the ability of parietal regions to inhibit behavior.
The strengths of our study include our whole brain analysis that was not restricted to a priori regions of interest, our large sample size, our patients with focal, rather than diffuse, lesions, and our homogeneous study population; however, this homogeneity (using only older men) makes the generalizability of the results to other populations uncertain. In addition, CT scans have less detail than MRIs, and the AIR registration process used in ABLe is a 12-parameter linear fit, which can lead to imperfect registration. In addition, pTBI can create positional shifts in brain tissue, as well as more chronic changes due to contraction in glial scars or the osmotic enlargement of fluid-filled spaces.
In conclusion, to our knowledge, this is the largest study of the neural correlates of behavioral disinhibition associated with focal brain lesions. Disinhibition is a debilitating problem leading to impulsive and socially inappropriate behavior. The identification of relationships between behavioral disinhibition and lesion location can help clinicians predict behavioral risk and develop coping strategies for patients and caregivers. On the positive side, the majority of veterans with pTBIs did not exhibit disinhibited behaviors. It is important to note that we have taken a snapshot in time and do not know whether they were disinhibited for a while after their injury but have since recovered.