Traumatic brain injury (TBI) is a major modern public health issue, which disproportionally affects young to middle-aged adults and is thus uniquely capable of robbing those injured of a lifetime’s worth of productivity in family, occupational, and social roles.
1 There is great interest in determining how to maximize functional recovery after TBI and in identifying factors that affect functional recovery.
Several injury-related measures are used to help predict the severity of TBI and outcomes, including the Glasgow Coma Scale score, time to follow commands, and duration of the post-TBI mixed confusional state, which is most commonly labeled posttraumatic amnesia (PTA).
2,3 This latter measure, first described in 1928 by Symonds
4 and later expanded upon by Russell and Smith,
5 has emerged as a strong predictor of TBI outcomes, surpassing the Glasgow Coma Scale in predictive value and leading some to call it the single best predictor of outcomes after TBI.
6,7 PTA has been shown to be a useful predictor of the following: subsequent functional level
8–10; cognitive recovery as assessed by neuropsychological test batteries,
11,12 measures of IQ,
13 driving skill,
14 and processing speed and executive function
15; subsequent unemployment at up to 5 years postinjury
16; overall cerebral atrophy
17; and social cognition as measured by facial emotion recognition
18 and tests of social inference perception.
19Although early studies used retrospective assessments to pinpoint patients’ emergence from PTA, current practice relies upon prospective measures such as the Galveston Orientation and Amnesia Test (GOAT) and the Orientation Log, which are designed for serial evaluation of orientation, recall, and new learning ability. Given the importance of PTA in predicting outcomes, the ability to predict its length at the time of initial injury, or at least at admission for neurorehabilitation, becomes potentially valuable as a means of connecting patients to appropriate interventions from the outset, framing expectations of patients’ families and anticipating care needs. In 1994, Katz and Alexander
20 examined multiple variables in a population of patients admitted for acute neurorehabilitation. They suggested that the length of PTA in weeks could be calculated as follows: 0.4 × length of coma in days + 3.6, although they noted that this relationship held true for patients with diffuse axonal injury but not those with focal contusions.
20 Sherer et al.
2 found predictive utility in the following formula: PTA (in days) = (1.8 × length of coma in days) + 24.8. In 2001, Tate et al.
21 proposed a three-variable model in which start date of PTA testing, rate of change of PTA scores over 5 days, and PTA score on day 1 of testing could predict 89% of the variance in length of PTA.
In this study, we aim to add to the limited body of literature examining possibilities for predicting length of PTA and functional recovery among patients with severe TBI. We focus on the patients who were still in PTA at the time of admission for acute neurorehabilitation and remained in PTA on average for >4 weeks.
Methods
Study Design
This is a retrospective study. We selected patients who sustained a TBI and were in PTA at admission for acute inpatient rehabilitation at the New York University Langone Medical Center Rusk Institute of Rehabilitation Medicine between January 2011 and December 2012.
Participants
A total of 40 patients were identified retrospectively through electronic chart review. Demographic details are shown in
Table 1. Selection criteria were as follows: age >18 years; recent nonpenetrating TBI; no prior history of cognitive, neurologic, or psychiatric disorder or substance misuse disorder; admission to New York University Langone Medical Center Rusk Institute of Rehabilitation Medicine for acute inpatient neurorehabilitation between January 2011 and December 2012; and in PTA at the time of admission (as defined by a GOAT score < 75).
Data Collection
Data were extracted from patients’ medical files in the New York University Langone Medical Center electronic medical record. Data were recorded on a standardized data collection form and were subsequently entered into a deidentified database. All information in the database was double-checked against the data collection form. From these data, we created a demographic table containing the following: age, sex, cause and date of injury, rehabilitation length of stay, and initial and discharge Disability Rating Scale, Functional Independence Measure (FIM), and Functional Assessment Measure (FAM) scores. We additionally collected data regarding brain lesion location, presence of seizures during the medical course, and use of neurostimulants.
Imaging Data Analysis
To collect data on lesion location, two neurologists performed independent evaluations of each patient’s initial computed tomography scan of the head performed on admission for acute care. Lesions were thus categorized into frontal, temporal, parietal, and occipital locations, and parietal lesions were further subcategorized according to laterality (right, left, or bilateral). These evaluations were then compared with each other and with the official radiologist’s read in order to reach a consensus on lesion location.
Main Outcome Measures
The GOAT was used to determine emergence from PTA. This scale was developed to evaluate cognition serially after a TBI, and it measures orientation to person, place, and time as well as memory for events before and after the injury. The 10-question test is intended to be administered daily. Its emphasis is on temporal orientation and recollection of biographical information. Interrater reliability is found to be excellent (correlation coefficient=0.99).
22 The GOAT has been used to determine the duration of PTA in numerous studies of TBI.
23,24 Patients admitted to acute rehabilitation in PTA based on the criteria set for the administration of the GOAT received daily administration of the assessment until they emerged from PTA, as defined by a GOAT score of ≥76 on three consecutive assessments. Length of PTA is defined as the number of days from initial injury until this point. Sixteen patients in our study did not emerge from PTA by the time of discharge from acute inpatient neurorehabilitation.
The FIM and FAM are primary functional outcome measures. The FIM is the most widely accepted functional assessment measure in rehabilitation settings (Guide for the Uniform Data Set for Medical Rehabilitation, 1996). The FIM is an 18-item scale, with each item score ranging from one (total assistance) to seven (complete independence). Scores below six indicate a requirement for another person for supervision or some level of assistance. The FIM measures independent performance in a range of domains, including self-care, transfers, locomotion, communication, and social cognition, and in doing so, the FIM also measures burden of care. By adding the points for each item, the possible total score ranges from 18 to 126 (indicating the lowest to highest levels of independence, respectively).
The FAM was developed as an adjunct to the FIM to specifically address the major functional areas that are relatively less emphasized in the FIM, including cognitive, behavioral, communication, and community functioning measures. The FAM consists of 12 items. Scoring for the FAM is identical to the FIM.
All patients admitted for and discharged from acute neurorehabilitation are routinely evaluated using FIM and FAM measures. FIM and FAM scores were obtained within 72 hours of admission and at the time of discharge for all participants. Rehabilitation team members were trained in the administration of the FIM and FAM and also had access to guidelines that assisted scoring. In addition to using composite FIM and FAM scores, scale items were divided into subscales of mobility and locomotion (FIM/FAM-motor), self-care (FIM/FAM-self), cognitive/communication (FIM/FAM-cognitive), and psychosocial adjustment (FIM/FAM-social).
Analysis
Intradepartmental statisticians reviewed the study design and performed all relevant statistical analyses (J.M. and A.B.). A regression analysis was performed to examine the relationship between PTA length and functional outcomes. A Pearson correlation was used to assess the relationship between the initial GOAT score and the length of PTA. Regression analyses were used to examine the relationship between lesion location and functional outcomes as well as PTA length. Analysis of variance was used to compare the groups of patients who received different types of neurostimulants to determine whether any particular stimulant had an effect on length of PTA.
Any analyses performed that did not directly involve PTA length included all subjects. Analyses that used PTA length as one of the variables excluded subjects who did not emerge from PTA before discharge. SPSS software (version 20 for Windows) was used for all analyses.
Results
PTA Length Is Associated With Functional Outcome
Consistent with extensive evidence in the literature,
9,25 we detected an association of PTA length with functional outcome. A simple linear regression was calculated to predict FIM discharge scores based on PTA length. A significant regression equation was found (F
(1,23)=7.247, p=0.013, R
2=0.240). PTA length significantly predicts discharge FIM scores (β=−0.530, p=0.013).
Initial GOAT Score Is Correlated With the Length of PTA
A Pearson correlation was used to examine the relationship between the length of PTA and the initial GOAT score on day 1 of admission for acute rehabilitation (p=0.031, coefficient=−0.432).
Initial GOAT Score Is Predictive of Overall Functional Outcome
A simple linear regression was calculated to predict FIM improvement scores based on the initial GOAT score. A significant regression equation was found (F(1,22)=6.918, p=0.015, R2=0.239). The initial GOAT score significantly predicts FIM improvement (β=−0.310, p=0.015).
Parietal Lobe Location of the Lesion Is Predictive of Functional Outcomes
We looked for any predictive value of the lesion location on either length of PTA or functional outcome.
Table 1 presents the pattern of lesion locations in our sample. Regression analyses were used to examine whether the presence of frontal, temporal, parietal, or occipital lesions is related to PTA length or discharge FIM/FAM scores. Frontal lobe lesions did not correlate with length of PTA or functional outcome (total FIM or FAM scores). Lesions in temporal and occipital lobes were similarly not predictive of PTA length or total FIM or FAM scores. We found that the presence of a parietal lobe lesion, however, is associated with worse discharge FIM (R
2=0.136, p=0.019) and worse FAM (R
2=0.096, p=0.050) scores. Parietal lobe lesions were also negatively correlated with most FIM and FAM subscales, including FIM-motor (R
2=0.152, p=0.013), FIM-self-care (R
2=0.229, p=0.002), FIM-cognitive (R
2=0.115, p=0.033), FAM-motor (R
2=0.133, p=0.021), and FAM-cognitive (R
2=0.101, p=0.046).
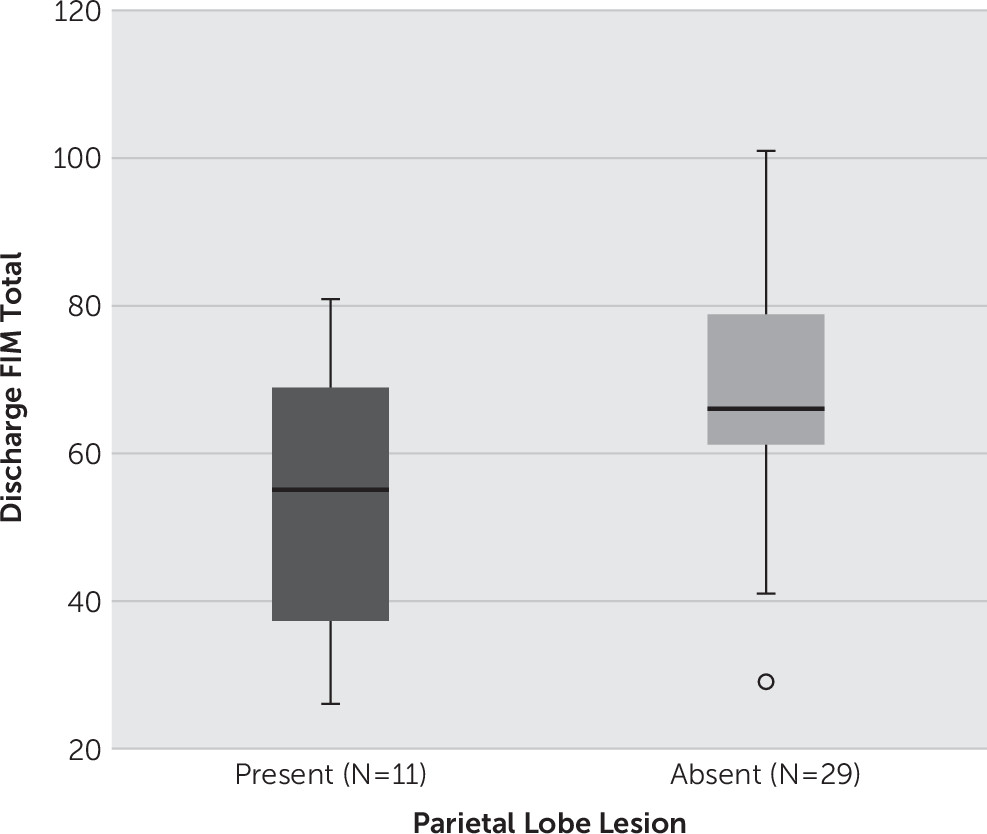
An independent-samples t test was conducted to compare discharge FIM scores for patients with and without parietal lobe lesions. There was a significant difference in the scores for no lesion in the parietal lobe (mean=68.0 [SD=16.62]) and lesion present in the parietal lobe (mean=53.1 [SD=18.90], t
(38)=2.44, p=0.019;
Figure 1). These results suggest that patients with injuries to the parietal lobe have a lower functional outcome measure at the time of discharge compared with patients without parietal lobe injuries. Similarly, discharge FAM was also worse for patients with parietal lobe lesions (t
(38)=2.01, p=0.051).
Multiple regression analysis was performed to examine the relationship between left, right, or bilateral parietal lobe lesions and functional outcomes. A significant regression equation was found (F(3,35)=4.301, p=0.011, R2=0.269). Right parietal lesions significantly predicted FIM change (β=−15.265, p=0.011), as did bilateral parietal lesions (β=−21.407, p=0.013). Patients who had only left-sided parietal lesions had no such relationship with functional outcomes (β=3.093, p=0.755).
We next examined whether the presence of visuospatial neglect may be driving the association between parietal lesion and outcome. Clinical data were consistent with visuospatial neglect in two of our patients: one had a right parietal lesion, and one had a bilateral parietal lesion. We repeated the independent-samples t test to compare discharge FIM scores for patients with and without parietal lobe lesions but with those patients excluded. There was now a trend but not a significant difference in discharge FIM scores for patients with no lesion in the parietal lobe (mean=68.0 [SD=16.62]) versus those with lesions present in the parietal lobe (mean=56 [SD=19.64], t(36)=1.79, p=0.083). These results suggest that visuospatial neglect may be driving the effect, although presence of a trend toward significance even with those patients excluded may suggest that other mechanisms may also be at play.
Neurostimulant Use Did Not Improve Outcome
Use of amantadine, methylphenidate, modafinil, or a combination of amantadine plus methylphenidate did not affect the length of PTA (
Table 1). An analysis of variance was used to determine whether neurostimulants improved rates of emerging from PTA. No such relationship was found with any of the neurostimulants. Averages of PTA length did not vary across neurostimulant categories, suggesting that no particular neurostimulant or combination thereof led to quicker emergence from PTA.
Discussion
There is no easy way to predict trajectories of recovery from TBI, particularly functional recovery. This study focused on patients with the most severe brain injury who were still in PTA at the time of admission for acute neurorehabilitation and remained in PTA for >4 weeks on average. The average length of PTA was 38 days among the patients in our sample who eventually emerged from PTA while in acute neurorehabilitation. By contrast, those who did not emerge from PTA during acute neurorehabilitation remained in PTA for >55 days. Our patients are categorized as having severe TBI according to commonly used stratification schemas for interpreting length of PTA, including the Russell and Smith
5 classification and the more recent Mississippi classification by Nakase-Richardson et al.
25Here we report several key findings in this patient population with severe brain injury. First, the initial GOAT score is correlated with the length of PTA and functional outcome. Second, the presence of a right parietal lobe lesion is predictive of worse functional outcome. Finally, the use of neurostimulants had no effect on the length of PTA or functional outcome.
Cognitive performance (as captured by the GOAT score) on the first day of acute rehabilitation provides information about the length of PTA and functional outcome (for scores on both the overall FIM as well as on cognitive portion). It will be important to determine whether this holds true for the Orientation Log as well, especially because there is currently a shift toward using the Orientation Log instead of the GOAT.
26 The GOAT was the primary PTA measure used in our facility during the years examined in this study and is thus the measure discussed here. To note, we recognize that the statistical significance of these results is not very strong, but the finding is there and the observation that an early first-day cognitive score (GOAT) can help predict outcome is important to report. Future studies in larger patient populations are needed, and the utility of the Orientation Log in predicting length of PTA and functional outcome in severe TBI must also be assessed.
The right parietal lobe emerges as a predictor of both motor and cognitive functional outcome. A role of the parietal lobe in recovery from TBI was hinted at in a prior study by de Guise et al.,
27 who found that the cognitive FIM score at discharge from acute care settings was better for TBI patients without parietal lesions, but no laterality-specific results were reported. Our finding is novel and can be interpreted along several lines of reasoning. It highlights the potential relationship between PTA and the attention system. PTA is increasingly being understood as related in part to attention system deficits, and parietal lobe injury may contribute by interrupting large-scale attentional networks.
28,29 There is also evidence that right parietal regions are part of a “when” pathway for both visual fields,
30 and sequencing of events may be particularly impaired in patients with right parietal lesions. In addition, there is evidence indicating that patients with neglect (which is common in patients with right parietal lesions) may have worse functional outcomes.
31 In our patient population, left visuospatial neglect was reported in two patients. Although neglect can be difficult to diagnose in patients in PTA unless it is profound and thus clinically unmistakable, our results suggest that the presence of a right parietal lesion even in the absence of clear neglect should alert us to the higher likelihood of poor outcome and prolonged PTA state. Patients with right parietal lesions may benefit from more aggressive individualized therapies for attention and neglect to help in functional recovery. We plan to address this in a future study.
Another mechanism by which parietal lobe lesions may contribute to worse outcomes in PTA patients may be through an effect on the memory system. The parietal lobe is involved in episodic memory recall, which may also play a role in PTA.
32 A loss of cognitive reserve may also be partly responsible for worse outcomes among patients with a right parietal lobe injury, because recent evidence points to the right hemisphere playing a role in cognitive reserve.
33 There are also some case reports from the 1980s describing acute delirium after right parietal lesions
34; in PTA, the right parietal lesion may contribute to a prolonged “delirium” state.
Although PTA has been the favored term for the post-TBI confusional period, debate on the nature of the cognitive deficits has been ongoing for nearly as long as the term’s existence; the debate particularly centers on whether the deficits observed are primarily the result of delirium/confusion versus episodic memory deficits versus attention impairment.
4,35,36 A recent study used the term
acute traumatic encephalopathy to stratify the cognitive deficits into categories of coma, posttraumatic delirium, PTA, and posttraumatic dysexecutive syndrome, which are conceptualized as occurring in temporal sequence after recovery of function in an ascending pattern from the brainstem toward the cortex.
37 This follows the hypothesis of biochemical and localizationist theories of emergence from disorder of consciousness, which involves recovery of function at the brainstem level first, followed by the diencephalon and then the telencephalon. Although this is an attractive model because of its logical conceptualization, challenges remain in disentangling where a patient may be on the spectrum from posttraumatic delirium to PTA.
No neurostimulant seemed to be better than others in terms of outcomes of PTA length in our sample of patients with severe TBI. As we learn more about how to classify patients based on the above-discussed criteria, we may be able to better target medications according to patients’ needs.
Understanding the underpinnings of the cognitive dysfunction seen in PTA as well as the determinants of emergence from this confusional period is crucial to advance our knowledge of the process of recovery from TBI. This is particularly relevant for patients with severe TBI, who invariably suffer lengthy periods of disorder of consciousness and/or PTA/PTC lasting from weeks to months.