Memory, the ability to maintain access to information in the absence of sensory input, is fundamental to all goal-directed behavior. Early models of memory were grounded in the logic models of the rising world of computer science and information theory.
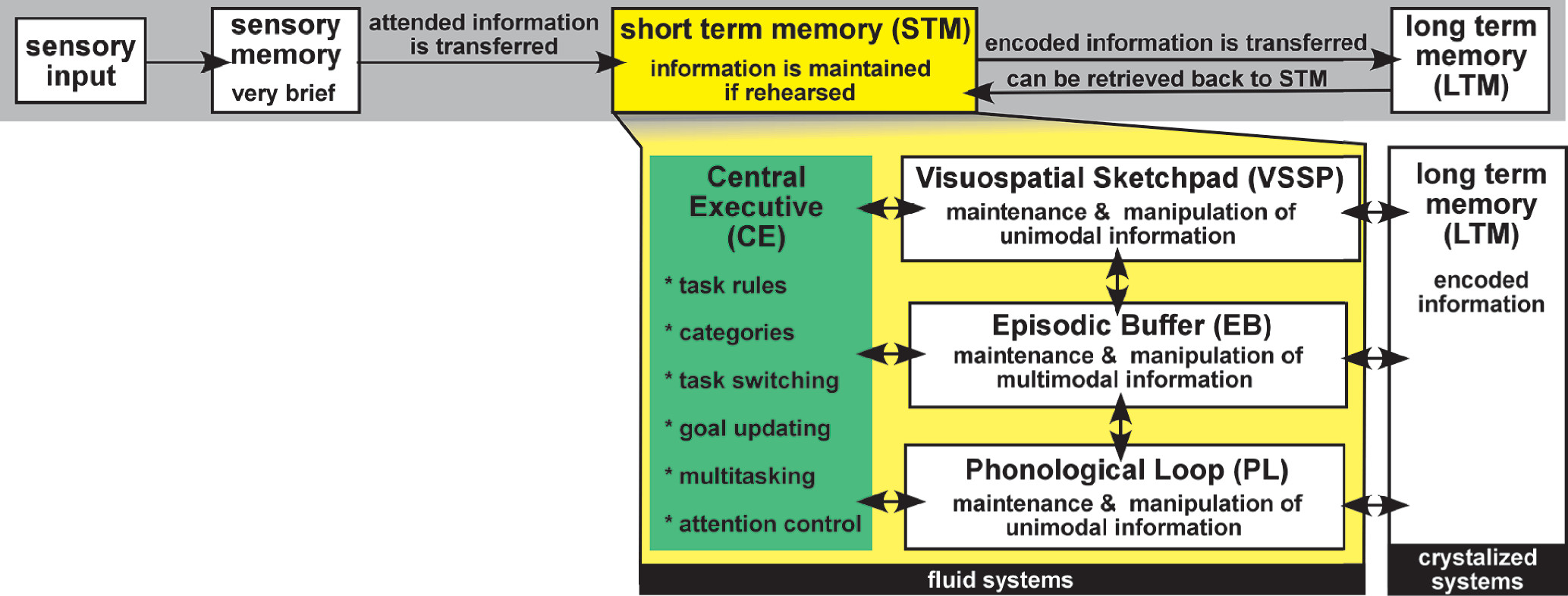
11 One popular model (Atkinson and Shiffrin Model) developed at that time proposed three stages of declarative memory: sensory memory (sense-specific registers), short term memory (STM), and long term memory (LTM). Information was lost if not successfully passed to the next stage (
Figure 1).
1 Although flawed, this model continues to provide a simple and easy approach to understanding memory. One assumption that proved to be inconsistent with research was that there is a single STM system. The low level of interference when concurrent tasks utilized different senses indicated that STM was not a unitary store. The multicomponent model of working memory (WM) essentially reconceptualized STM by expanding it into more than one component and adding a set of active processes (
Figure 1).
2 This model of WM continues to be the most widely used conceptual map.
12–17 The original publication has been cited nearly 10,000 times in more than 40 years since its initial presentation.
16 Due to the importance of intact WM for daily functioning, foundational understanding of WM theory, tests, and recent neuroimaging is of value for both researchers and clinicians working in neuroscience-related fields.
Neuropsychology
The concept of intelligence provides a context to understand other cognitive abilities. Intelligence is generally defined as general mental capacity. There are numerous theories of intelligence, with many including WM as a component. The Cattell-Horn-Carroll (CHC) model of intelligence, a comprehensive and widely accepted theory, is hierarchical.
18,19 General intellectual ability (
g) encompasses the comprehensive representation of global intelligence. Within
g are various broad abilities (e.g. fluid intelligence [mental efficiency] and crystallized intelligence [acquired knowledge]), each of which can be further segmented into numerous narrow abilities.
20 Although the model encompasses a STM component, WM is not directly incorporated. However, abilities such as general sequential reasoning (under the fluid intelligence factor) and memory span (under the general memory and learning factor) require use of WM. Thus, WM is represented in (but not actually part of) the model. Numerous studies have found moderate to high correlations between WM and
g.
21 Two mainstream intelligence tests (Stanford-Binet and the Wechsler Intelligence Scales [WIS]) directly incorporate WM into their batteries, comprising a full scale intelligence quotient (IQ score). The relationship between WM and intelligence is partially mediated by how intelligence is measured. Of particular note, the WIS Working Memory Index includes Digit Span, Arithmetic, and Letter-Number Sequencing, three commonly used tests in both clinical and research settings. As the WIS are some of the most popular tests of intellectual ability in current use, many IQ estimates will inevitably be greatly influenced by WM ability.
Although conceptualized as an active STM process, WM tasks are commonly classified as attentional in neuropsychology. One major text introduces WM within the more global memory paradigm but describes tests of WM as the combination of storage and manipulation of information.
22 WM tests are included among tests of attention, not with tests of memory. Commonly used WM tests do not directly correlate to the multicomponent WM model. WM tests are classified generally, or at most, divided by modality (visual versus verbal). In general, WM tests can be grossly divided into two categories: complex span and complex tracking. Additionally, there is a division between those tests commonly used in research and those commonly used clinically. One study assessed whether research WM tests (operation span, listening span, and N-back) were consistent with those used clinically (WIS and Wechsler Memory Scale tests).
23 The three research tests and WAIS-III Letter-Number Sequencing were the most strongly correlated. The model containing those four measures provided the best fit to the latent WM variable. Thus, WIS Letter-Number Sequencing (a complex span test) may be a common metric for translation across the settings.
Two commonly used tests of complex tracking are Paced Auditory Serial Addition Test (PASAT) and N-back. During the PASAT, single-digit numbers are presented at various time intervals and the respondent must add each number to the immediately preceding number, though there are a number of versions of the test available.
24 The PASAT is frequently included in fixed multiple sclerosis batteries due to its sensitivity to impairments common to that disorder.
25,26 Psychometrically, the task seems to load heavily on processing speed, attention, and executive functions.
27 Although the test is difficult and many find it aversive, it was reported to be the third most popular test of attention among neuropsychologists according to one survey.
28 The N-back paradigm is a category of complex tracking tests that is frequently used in neuroimaging studies. A series of stimuli (verbal or nonverbal) are presented, requiring the respondent to signal when the new stimulus matches the one presented “n” items before. Thus, this test requires both WM and vigilance. Increasing the span (or “n”) increases the complexity of the test. The stimuli can be numbers, letters, shapes, or location of a stimulus in space.
In contrast, complex span tests focus more on attentional capacity. A commonly used test is Digits Backward. Strings of digits are presented with the respondent reciting the digits in reverse order. On subsequent trials the length of the string (digit span) is increased. The test is heavily researched, easy to administer, and a regular subtest in the WIS. Current versions combine Digits Backward with the simpler Digits Forward and the Digit Sequencing tests. However, factor analyses indicate that simple span and complex span tasks are related but not equivalent.
29 Digits Backward is an ideal test for use as part of a bedside exam due to its ease and speed of administration. A Digits Forward span should be completed first. The typical forward span for adults is seven digits; the typical backward span is four or five digits correct.
22,30,31 Numerous other complex span tasks are in use (e.g. reversed spelling, mental arithmetic, WIS Letter-Number Sequencing). The Corsi block tapping test, has been computerized, making it useful for neuroimaging studies.
32 The stimulus is an array of 3D blocks arranged in space. The examiner taps the blocks in a particular order, with the respondent then tapping the blocks in the same (or reverse) order. On subsequent trials, the number of taps increases (similar to digit span). A series of dual procedure and suppression experiments using the Corsi forward and backward paradigms found that the Corsi test employs VSSP and CE but not PL, suggesting that the Corsi test fits well with the multicomponent model of WM.
33 The importance of this finding is that most WM tests employ PL and CE, with fewer tests available to elicit VSSP.
Neurobiology
The classic approach for identifying the neural architecture supporting particular aspects of function is the study of patients with focal brain lesions.
2,13 Studies that establish correlations between morphological and performance variables (i.e. lesion-deficit analyses) provide a basis for identifying areas of the brain that support specific abilities. As intelligence and WM are strongly interrelated, the most informative studies include a sufficiently broad range of performance measures to support assessment of both. A recent series of studies utilized voxel-based lesion-symptom mapping (each voxel designated as intact or lesioned) to delineate the neural architecture underlying the major types of intelligence (general, fluid) and major components of WM (domain-specific information maintenance, information monitoring, information manipulation, cognitive flexibility).
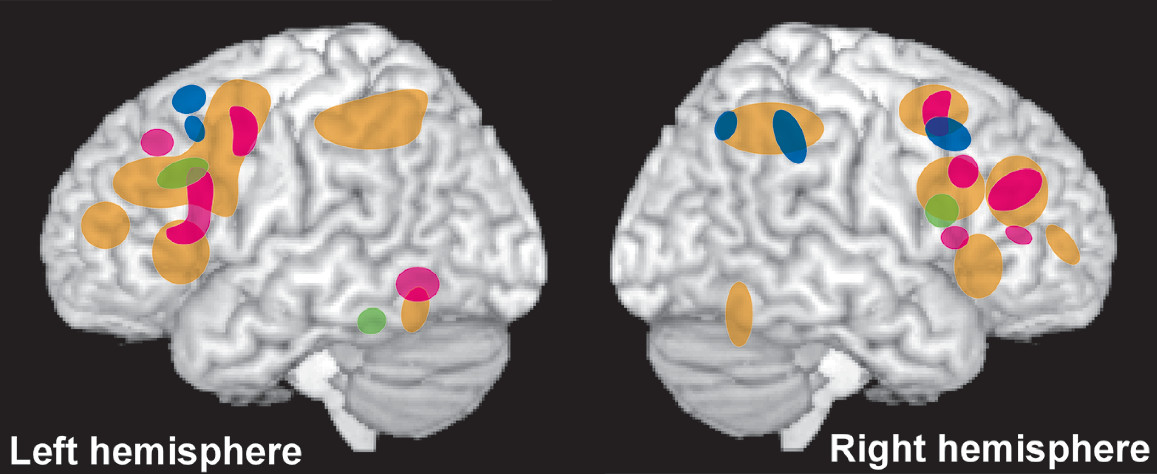
3,4,6 Both general and fluid intelligence were associated with widely distributed networks of cortical areas (ventrolateral and dorsolateral prefrontal [PFC], primary motor and somatosensory, inferior and superior parietal, and superior temporal cortices) and associated major white matter tracts. General intelligence was primarily left lateralized, whereas fluid intelligence was primarily right lateralized (
Figure 2).
3,6 The areas associated with general intelligence were similar in locations but greater in extent than reported in a previous study.
34 Domain specific WM lateralized by domain (
Figure 2), with verbal/ numeric primarily associated with areas in the left hemisphere (dorsolateral PFC, superior parietal and superior temporal cortices, angular gyrus) and spatial primarily associated with areas in the right hemisphere (dorsolateral PFC and superior parietal cortices).
6 Both overlapped with areas associated with intelligence in dorsolateral PFC and superior parietal cortices.
3,6 Cognitive flexibility and the information manipulation aspect of WM were both associated with primarily left hemisphere networks of cortical areas (
Figure 2). Cognitive flexibility overlapped areas associated with general intelligence throughout that network.
3,4 Some of the left hemisphere areas associated with information manipulation overlapped with areas associated with general intelligence (superior parietal and superior temporal cortices), whereas others (angular gyrus) did not.
3,6 In addition, some areas in the right hemisphere associated with fluid intelligence were also associated with information manipulation (dorsolateral PFC). The information monitoring aspect of WM was associated with a primarily right hemisphere network of cortical areas (ventrolateral PFC, inferior parietal and middle temporal cortices) that overlapped the fluid intelligence network only in inferior parietal cortex.
6Studies in healthy individuals are also informative. A meta-analysis of the association between PFC volume and thickness and tests of executive functioning found a moderate effect size for volume and a weak effect size for thickness.
35 In the regional analysis, both lateral and medial PFC volumes were significantly correlated with executive functioning.
35 Similarly, larger gray matter volume was associated with better executive performance both at the global (whole brain) and regional (PFC, insular and cerebellar clusters) levels in another study.
36 A study quantified several measures of cortical morphology (gray matter volume, cortical surface area, cortical thickness) to identify areas supporting aspects of intelligence (fluid, crystalized, spatial) and basic cognitive processing (WM capacity, executive updating, attention, processing speed).
37 Statistical parametric mapping of gray matter volume indicated that all three intelligence factors were associated with partially overlapping clusters in right dorsolateral PFC. Spatial intelligence was also associated with clusters in right inferior temporal cortex (fusiform gyrus) and caudate. WM capacity was associated with a cluster in right dorsolateral PFC that overlapped fluid and crystalized intelligence. WM was also associated with clusters in right primary somatosensory cortex and left frontal and occipital cortices. Attention was associated with clusters within frontal, temporal and occipital cortices.
37 A study that estimated size of visual cortex using both structural (landmark based parcellation) and functional (retinotopic mapping) measures found that the size of V1 (first stage of visual processing) correlated strongly with visual WM capacity.
38 As noted by the authors, these findings suggest that individual differences in the size of domain-specific cortices may contribute to individual differences in domain-specific WM performance.
Functional imaging provides a range of methods for probing the neural architecture supporting particular aspects of brain function. Task-activated functional imaging studies are most commonly used to identify areas of the brain that are more active during performance of a particular task. Most functional imaging studies utilize functional magnetic resonance imaging (fMRI), which provides an indirect measure of neuronal activity based on local changes in blood flow. The majority of studies compare signal intensity of relatively large groups of voxels (cluster or region) across conditions (univariate analyses). Multivariate pattern analytic (MVPA) techniques have recently been introduced that utilize machine learning techniques to identify patterns of activated voxels that encode task-related information. Comparisons across types and phases of tasks have the potential to provide insight into the brain areas supporting different components of WM. One example is comparing tasks that require similar manipulations of information but utilize different information domains. Processes related to maintaining domain-specific information (e.g. visual, verbal) might be expected to localize differently, whereas domain-general processes (e.g. remembering rules or goals, ignoring distractors) might be expected to localize similarly.
Several recent meta-analyses of functional imaging studies reported bilateral activation of a network of frontal, parietal, temporal, cerebellar, and thalamic areas across WM tasks (e.g. N-back, Sternberg, delayed matching to sample, prospective memory) and stimuli types (
Figure 3).
7–10 The meta-analysis that compared by gender also found a few differences, such as greater activation of limbic areas in females and parietal areas in males.
8 Increasing WM load (increasing task difficulty or adding the presence of distractors) was associated with increased activations in almost all areas (exceptions were rostral lateral PFC and thalamus), suggesting a network supporting domain-general executive control processing (
Figure 3).
7 A study utilizing MVPA reported successful cross-modal (visual WM task, verbal WM task) prediction of WM-load-sensitive areas, further supporting a common domain-general neural substrate.
39 A study utilizing graph-based modularity analysis found that WM tasks activated multiple areas within the executive control (lateral PFC, superior parietal lobule) and salience (anterior insular and dorsal cingulate cortices) networks.
40 Connectivity within the executive control network and between the executive control network and salience network increased with increasing WM load.
40Some areas, most commonly within lateral PFC and parietal cortex, display sustained activation during the delay period in WM tasks.
12,13,17,41,42 One interpretation has been that this represents domain specific information being maintained in an active state. However, fMRI studies utilizing MVPA have reported that patterns of activity in lateral PFC contain higher order information required for task performance (e.g. task rules, stimulus-response mapping, categories based on multiple domains of information) rather than domain specific information.
12,13,41,42 These findings support the alternative view that sustained activity in lateral PFC and parietal cortex indicates allocation of cognitive resources (e.g. attention) to maintain domain specific information elsewhere (top-down modulation). Congruent with this view, MVPA studies identified patterns of activity in sensorimotor cortices during delay periods consistent with population encoding of domain specific information even in the absence of elevated activity.
12,13,41,42 Patterns were predictive across memory types (WM, LTM), suggesting a common neural substrate for WM and LTM. An fMRI study using MVPA reported dynamic shifts in patterns of activity when a retrocue (cue presented when the stimulus is no longer available) signaled a change in the stimulus dimension required for task performance.
43 This suggests that what is encoded is not a static representation of all information contained in the stimulus, but rather specifically favors task-relevant aspects.
The multicomponent model of WM is a conceptual model, informed by and developed based on cognitive psychology research. Advances in identifying neural correlates of processes that contribute to WM have provided insights into the underlying neural architecture. For example, in the multicomponent model WM and LTM are functionally distinct systems. There is growing evidence from neuroimaging research, particularly MVPA studies, that WM and LTM are neuroanatomically colocated. This apparent disparity has been reconciled in state-based models of WM, which postulate that these types of memory differ by activation state rather than location in the brain.
12,13,15,17,41 In these models, attention is the key factor determining the accessibility of memory content. Terminology and number of activation states varies, but the basic postulates are similar. WM contains information that is the focus of attention (region of direct access). Other information ranges from less accessible (latent WM, activated LTM) to not directly accessible (LTM). The hierarchical process model also incorporates timescale and information complexity to distinguish the nature of memory processing in domain-specific areas from that occurring in higher order areas.
15