Posttraumatic stress disorder (PTSD) can occur following events considered to be traumatic. The disorder is characterized by re-experiencing symptoms relating to the trauma, mood changes, avoidance symptoms, and features of hyperarousal, which are persistent and cause significant reductions in functioning.
1 A number of studies have investigated structural brain changes in PTSD utilizing magnetic resonance imaging (MRI). Findings to date have included reduced bilateral hippocampal volume
2–4 and a decrease in the volume of the pregenual anterior cingulate and subcallosal cortices.
5 MRI studies have produced mixed results in regard to amygdala volume. A study of cancer patients with subthreshold PTSD characterized by recurrent intrusive memories
6 found smaller amygdalae, particularly on the left side, by comparison with a control group. Karl et al.,
7 in a meta-analysis of 11 relevant studies published up to 2006, concluded that PTSD patients had smaller left amygdalae than controls. Woon and Hedges
8 conducted a meta-analysis of nine studies, excluding pediatric data, and found no difference in amygdala volume compared with controls for adults with PTSD. In studies of veterans with PTSD, Kuo et al.
9 showed increased total amygdala volumes in their PTSD cohort by comparison with controls and a negative correlation between trauma history and amygdala volume. Morey et al.,
10 however, found decreased bilateral amygdalae in the PTSD group and no correlation with trauma load.
Eye movement desensitization and reprocessing (EMDR) and prolonged exposure (PE) are well-established first-line psychological treatments for PTSD.
11–13 However, key differences have been noted in the underlying processes. Unlike PE, EMDR does not involve repeated detailed descriptions of the event, and the exposure is not of an extended duration.
13 It has been argued that EMDR does not work through the processes of extinction as does PE.
14 Indeed, processes that have been found crucial in PE and linked to extinction, such as having the client relive his or her trauma experiences,
15 have not been found relevant in a positive response to EMDR treatment.
16 Instead, the short exposures associated with EMDR treatment may mean that EMDR works through a reconsolidation process. Research has shown brief exposures are associated with reconsolidation, while prolonged exposures are associated with extinction.
17 Furthermore, reconsolidation and extinction are two opposing processes following memory retrieval, which have distinct biochemical signatures.
17 Finally, extinction appears to be mediated by the prefrontal cortex,
18,19 whereas reconsolidation of fear memories is thought to more directly involve the amygdala.
20,21There is some limited initial evidence that successful treatment of PTSD could produce measurable structural changes in brain regions associated with fear conditioning. Several studies have reported significantly larger hippocampal volumes following treatment of PTSD with medication
22–24 and psychotherapy.
25 Quidé et al.
26 conducted a systematic review aimed at comparing the effects of psychotherapy and pharmacotherapy on brain structure and function in anxiety disorders and major depressive disorder. The authors’ principal conclusion was that psychotherapy tends to increase activity and recruitment of frontal areas (top-down effect), while pharmacotherapy decreases overactivity of limbic structures (bottom-up effect). However, no distinction was made between different types of psychotherapy used, and there were very little data available relating to impact of treatment on amygdala volumes. In a separate study, Levy-Gigi et al.
4 found no change in amygdalae volume following a short course of trauma-focused cognitive-behavioral therapy (CBT). However, this study did not include any PE in the CBT treatment. In regard to neurobiological changes following treatment with EMDR, there are no published data regarding amygdala volume. One small, uncontrolled study found significant increase in hippocampal volumes after 12 EMDR sessions but did not report on the amygdala.
27The aim of our study was to examine structural changes in both the amygdala and hippocampus before and after treatment with either EMDR or PE. We chose EMDR and PE as they have been associated with large treatment effect sizes in PTSD.
11 We chose to examine the hippocampus given the existing evidence that it is reduced in volume in PTSD and may increase following effective treatment, and we chose the amygdala given the evidence for its strong role in fear conditioning and increased activation in PTSD.
28 We expected to find significant changes in amygdala and hippocampal volume following treatment.
Methods
Participants
Twenty patients were recruited from consecutive referrals to the Posttraumatic Stress Clinic at the Alma St. Psychiatric Unit, Fremantle Hospital, and the Psychology Clinic at Murdoch University. Approval for the study was obtained from the South Metropolitan Hospitals Ethics Committee and the Murdoch University Ethics Committee. The inclusion criteria were that participants be between 18 and 65 years old, meet Clinician Administered PTSD Scale (CAPS)
29 criteria for PTSD, and be willing to engage in psychological treatment and neuroimaging. Patients included in the study were not to have initiated any new prescription medication within the previous month. Patients with current drug or alcohol dependence, diabetes, past history of psychotic illness, neurological disorders, evidence of a diagnosable cluster B personality disorder scored via the Structured Clinical Interview for DSM-IV Personality Disorders (SCID II),
30 and a score greater than 30 on the Dissociative Experiences Scale (DES)
31 were excluded.
Forty-eight patients were screened for participation, and 20 patients were recruited into the study. Patients were excluded from the study due to failure to meet PTSD diagnostic criteria (N=5), inability to complete baseline brain scans (N=4), evidence of significant dissociative symptoms (N=3), evidence of cluster B personality disorder or previous brain injury (N=6), declining to participate after assessment (N=5), and other reasons (N=5). Demographic information for the 20 participants is summarized in
Table 1. Briefly, the sample consisted of 14 (70%) females and six (30%) males, and the mean age was 40.1 (±9.9) years. Within the PE group, four participants were taking no psychotropic medication, six were taking an antidepressant, and one was prescribed a benzodiazepine. In the EMDR group, three participants were taking no psychotropic, six were prescribed antidepressants, and three were prescribed an antipsychotic agent (for treatment of PTSD symptoms). Participants were randomly allocated to either EMDR or PE using a computer-generated random number sequence that was set to allocate 10 patients to each condition without restrictions. Each participant was allocated to treatment after all baseline data were collected by the third author, who had no contact with the participants nor any indication of their baseline scores. All 20 patients completed the study protocol.
Measures
Clinical assessment included the following instruments: CAPS, SCID II, and DES. The CAPS was the primary measure of clinical symptom change and was administered at baseline and posttreatment by a rater who was blind to the treatments received and imaging results. The CAPS had excellent internal consistency in this sample, with coefficients between alpha=0.90 and alpha=0.97. The SCID II and DES were administered at baseline only, as their purpose in this study was to identify exclusion criteria (see above).
Clinical Intervention
Participants in both EMDR and PE therapy received twice-weekly therapy sessions for 12 sessions from a mental health clinician trained and experienced in the therapy. Both therapies were carried out according to standard, internationally accepted protocols. PE was structured in accordance with the Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences-Therapist Guide,
32 whereas EMDR was based on the Eye Movement Desensitization and Reprocessing: Basic Principles, Protocols, and Procedures.
33 Each of the two therapists involved in the study attended training in both therapies, and each therapist provided treatment to half the patients in the EMDR group and half the patients in the PE group. The PE training was accredited by the Australian Centre for Posttraumatic Mental Health and the EMDR training by EMDR International Association Australia. Supervision was provided for both treatments, and fidelity ratings were completed by an independent senior therapist utilizing video recordings of sessions to ensure the therapy met these standards.
MRI
All subjects underwent MRI imaging using a standard multimodal protocol on two occasions: pretreatment and after 12 sessions of treatment. The mean delay from completion of treatment to the second MRI was 23 days (26 days for the PE group and 19 days for the EMDR group). All scans were carried out in a standard fashion on a Siemens Vision 1.5T scanner at Fremantle Hospital, and the data were stored electronically. The following sequences were obtained: three-dimensional T1-weighted (three-dimensional-MPRAGE: echo-time [TE]=3.34 ms; repetition time [TR]=2,530 ms, inversion time [TI]=1,100 ms, flip angle=10°, field of view [FOV]=26.0, matrix=256×256×192 mm, no gap [slice thickness 1 mm]); a T2-weighted sequence (TE=75 ms, TR=7,800 ms, FOV=26.0, matrix=256×256, no gap [slice thickness=1 mm], turbo factor=9).
Imaging data were analyzed at the Neuroimaging Laboratory at Fremantle Hospital using BRAINS-2 software. Image analysis was carried out by S.S. and S.B., blind to both treatment condition and clinical findings. All scans were processed through an automated method reoriented by stepwise coregistration to a set of template images, centered on the anterior commissure, and resampled to 1-mm resolution. All images were intensity normalized and inhomogeneities were corrected. A discriminant tissue classification was performed and a brain mask created using an artificial neural network (ANN).
34 Measures of gray matter, white matter, and CSF volume were compared using the standard Talairach method. ANNs were applied to each scan to measure cortical and subcortical structures, including the amygdalae. Results of this procedure were visually inspected by a neuropsychiatrist with experience in using BRAINS-2 (S.S.), and all scans passed all control stages successfully. Volumes are reported in cubic millimeters. To control for differences in overall head size, all measures were analyzed as a proportion of intracranial volume (ICV).
The methodology, validity, and reproducibility of morphometric analysis have been reported by our group (RP4, SS25).
35Data Analysis
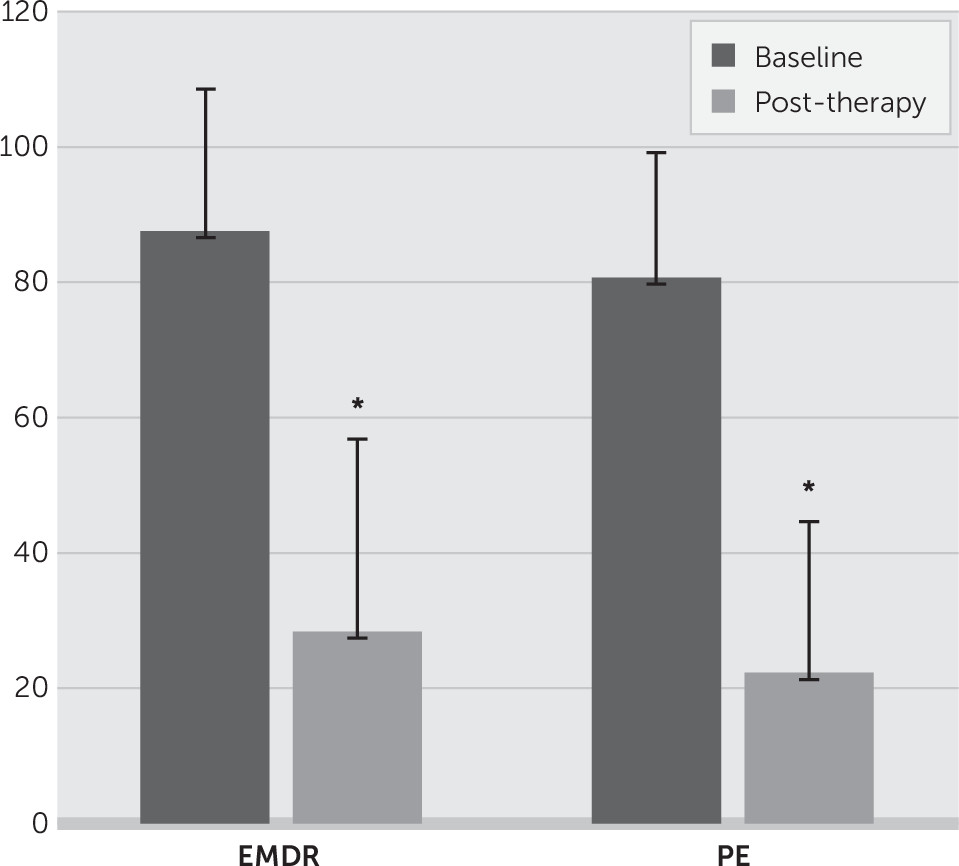
Data were analyzed using the SPSS Statistics 22 software. There were no missing data. Normalized amygdala and hippocampal volumes pre- and posttreatment were analyzed by time (before and after treatment), side (left and right), and treatment group (EMDR, PE) using multivariate repeated-measures analysis of variance. A significant interaction was explored further with planned contrasts (paired t tests) within each group of left versus right amygdala and hippocampal volume at pretreatment and posttreatment. Clinical change via CAPS was assessed in similar analyses, with contrasts between the baseline assessment (6 weeks before treatment) and the pretreatment assessment and between pre- and posttreatment assessments.
Discussion
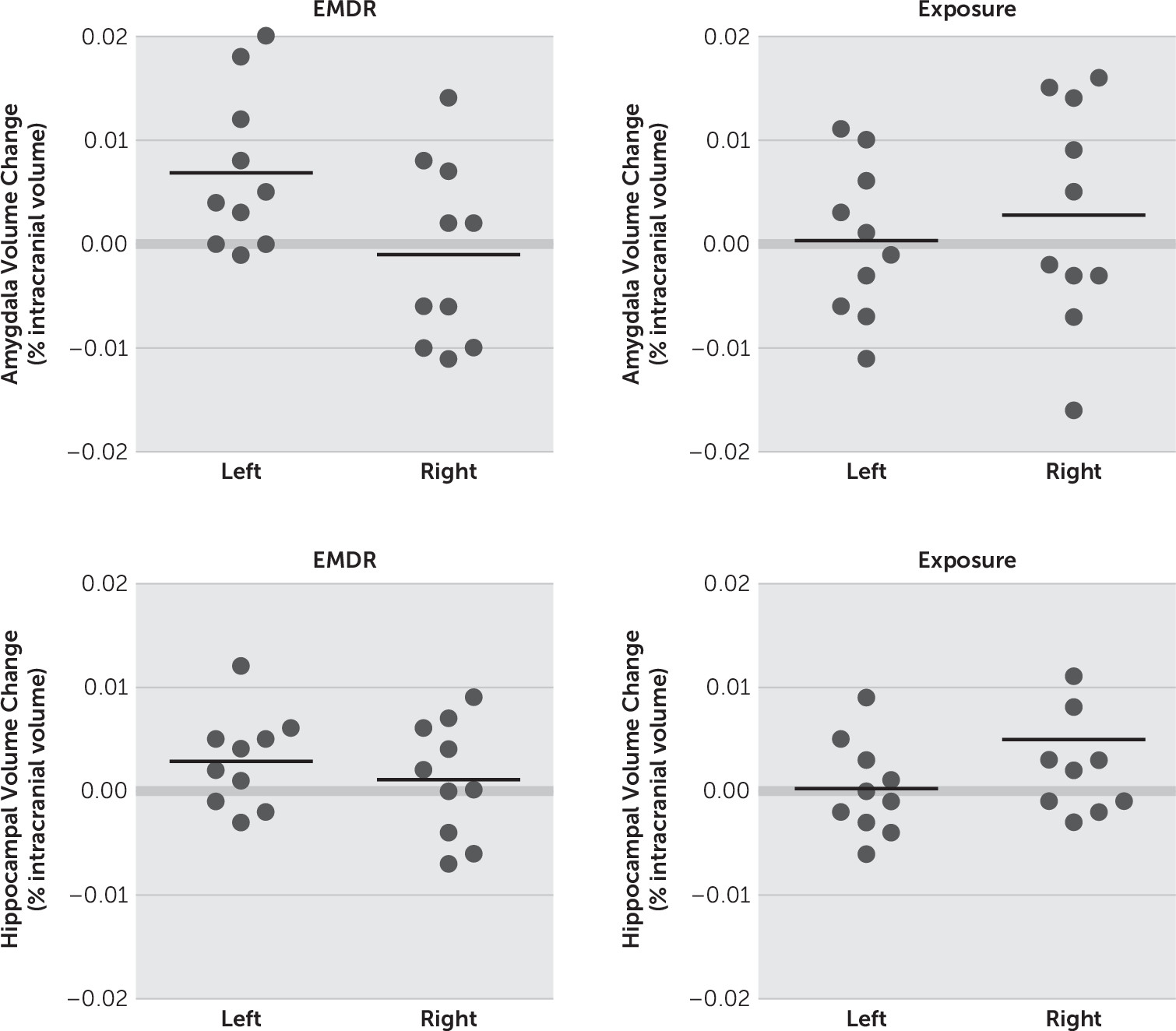
We investigated the hypothesis that structural change may occur within the hippocampus and amygdala following two accepted psychotherapeutic interventions. Significant treatment effects were demonstrated for each of the interventions, which is consistent with existing evidence. We found evidence for volumetric increase for the left amygdala only after 12 EMDR sessions but no similar increase following 12 sessions of PE. No significant change in hippocampal volume was found following 12 sessions of EMDR or PE. We did not, therefore, replicate the finding of increased hippocampal volume following EMDR reported by Bossini et al.
27 These findings are intriguing in a number of ways and may indicate a difference in the underlying biological mechanisms for these two therapies. These data raise the possibility that PE operates by modulating regions other than the amygdala implicated within the fear conditioning network. The literature suggesting different underlying processes for the efficacy of EMDR and PE has been summarized in the introduction,
13–17 and there is some evidence for possible differences in key brain regions implicated in these processes.
18–21There is good evidence for increased activation of the amygdala in PTSD when the traumatic memory is reactivated. Successful treatment of PTSD would therefore be expected to produce a decrease in activation of the amygdala from a functional perspective. The questions therefore arise as to why symptomatic improvement should lead to an increase in amygdala volume, and why in the left amygdala rather than the right? We cannot answer these questions with any certainty from the current data, but the existing literature may indicate some possible mechanisms. Previous MRI studies have indicated that total amygdala volumes in patients with PTSD can be increased
7 or decreased
8 by comparison with controls. In a review of the relevant literature, including both human and animal studies, Tottenham and Sheridan
36 suggested that timing of trauma in relation to developmental stages may be important in understanding some of the apparent inconsistencies. These authors suggest that amygdala volume increases secondary to early-life traumas but may atrophy while remaining overactive later in adult life. Our findings suggest a possible reversal of this atrophy following EMDR, but no clear mechanism for this can be shown from the data.
The question of laterality is also intriguing. In a meta-analysis of 82 studies, Pedraza et al.
37 found that the amygdala is reliably asymmetrical in the normal adult population, with the left amygdala significantly smaller in volume than the right amygdala (p<0.001). In regard to clinical populations, a Japanese study of 76 cancer survivors compared those who experienced cancer-related intrusive recollections with those who did not.
6 The authors noted that although the patients were not diagnosable with full PTSD, the presence in such patients of clinically significant intrusions can be considered characteristic of subthreshold PTSD. Furthermore, total amygdala volume was smaller in patients with intrusive recollections compared with the control group, and the volume reduction for the left amygdala was twice that for the right amygdala.
6 In their meta-analysis of amygdala volume in adult PTSD patients, Woon and Hedges
8 found significantly reduced left amygdala volumes, as compared with the right, in both the patient group and the controls (subjects unexposed to trauma and those exposed to trauma but without PTSD). These findings suggest that asymmetric lateralization of amygdala volume in normal unexposed subjects is preserved in trauma exposure and in PTSD. There is, then, support within the existing literature for amygdala volumetric lateralization in the nontraumatized, the traumatized without PTSD, and the PTSD population with smaller left-sided amygdalae, which may be further reduced in volume in PTSD patients.
The main limitation of this current study is the relatively small number of participants in each treatment group, and this invites caution in interpreting the findings. Thus, the lack of volumetric change in the PE group may have been related to limited study power rather than absence of treatment effect. However, there is very little previous evidence regarding the impact of effective psychotherapy for PTSD on the amygdala and hippocampus, and no previous study has compared EMDR and PE in this regard. Another possible limitation is that the study excluded participants with severe dissociative symptoms and cluster B personality disorder. While this helps to assess the effects of treatment in a more pure PTSD sample, the findings may not generalize to PTSD samples that include these diagnostic features. This could be tested in future research.
A possible limitation which may be relevant is that patients in the study were not randomized for trauma type. Of particular note is that five patients in the EMDR group had primary sexual traumas, compared with none in the PE group (
Table 1), and this may be an important confounding factor, which future studies should address. However, the literature does not support that type or severity of trauma makes any difference to treatment outcome. For example, in one of the first studies to examine this issue, no difference in treatment outcome was found for participants whose trauma consisted of childhood sexual abuse, adult sexual assault, or adult nonsexual assault.
38 In a later meta-analysis involving 13 studies and 675 participants, it was found that trauma history, including type of trauma and whether or not there was repeated traumatization, did not have any significant effect on the outcome of treatment.
39 Since the meta-analysis, a large study of 110 female PTSD participants also found that prior trauma severity did not impact on treatment. For example, a history of childhood sexual abuse in addition to trauma was not associated with the severity of the initial PTSD symptoms, symptom reduction during the treatment study, the rate of change during treatment, or the number of sessions needed.
40 However, just because type of trauma does not affect treatment outcome does not rule out that it could differentially affect brain functioning. This is an empirical question that is yet to be tested, to our knowledge, and further research could investigate whether this is possibly the case.
The relative strengths and weaknesses of an automated image analysis approach versus a manualized approach may be debated. A semiautomated system, such as BRAINS II, avoids the confounding factors inherent in manualized tracing. Furthermore, there is evidence from a study measuring hippocampal volume in PTSD patients that BRAINS II findings are consistent with an alternate method using voxel-based morphometry.
41Our results, while preliminary, add to the existing literature in suggesting a possible difference between these two established PTSD treatments at a biological level. These findings indicate the value of further studies with larger subject numbers. Such studies with a larger sample size could also investigate clinical severity and dissociation scores at baseline as predictors of amygdala volume outcomes posttreatment and the differential effects of trauma types on amygdala volume.