Tics and Tourette’s Syndrome
Tics are relatively common symptoms of hyperkinetic movement disorders and are defined as involuntary, sudden, rapid, recurrent, nonrhythmic movements (motor tics) or vocalizations (vocal/phonic tics).
1 From a clinical perspective, the most relevant tic disorder is Tourette’s syndrome (TS), first described by Georges Gilles de la Tourette in 1885
2 and currently diagnosed based on the chronic presence of at least two motor tics plus one vocal tic with onset before the age of 18.
3,4 The modern scientific literature on TS has been dominated by clinical descriptions of tics as viewed from an external perspective, therefore focusing on their objective features (anatomical location, number, frequency, intensity, complexity, duration). The structure of available clinical rating instruments for tics reflects this perspective. For example, the Yale Global Tic Severity Scale (YGTSS), the most widely used instrument to rate tic severity in clinical trials includes separate ratings of the number, frequency, intensity, complexity and interference associated with motor and vocal tics, plus an overall impairment rating.
5 Patients’ first person perspectives on their tics can reveal intriguing features, which are key to the clinical phenomenology of these symptoms. Specifically, patients often report that their tics are preceded by a subjective feeling of “inner tension of wanting to move” and that this unpleasant feeling is only temporarily relieved by tic expression.
6–9 The presence of such sensory symptoms or “premonitory urges” to tic is useful in the differential diagnosis between tics and other repetitive behaviors, such as myoclonus, functional jerks, stereotypies and mannerisms.
Patients with tic disorders commonly report that their urges to tic can be resisted for a finite length of time, at the expense of mounting inner tension (tic suppressibility).
10 The exact pathophysiology and brain mechanisms underlying tic expression are not fully understood, however converging evidence points toward a role for dysfunction of dopaminergic transmission within cortico-striato-cortico-frontal circuitries as a key etiological pathway.
11,12 TS is a genetically heterogeneous condition, with epidemiological evidence suggesting an association with pre- and peri-natal problems and a possible role for poststreptococcal autoimmune dysfunction in the etiopathogenesis of at least a subgroup of patients with TS,
13,14 although this remains highly controversial. Treatment approaches for tic symptoms are in line with our understanding of their pathophysiology: pharmacotherapy mainly relies on the use of antidopaminergic agents
15 and deep brain stimulation for severe refractory cases target specific components of the cortico-striato-cortico-frontal loop (especially globus pallidus and nonspecific thalamic nuclei).
16 Over the last decade a growing body of research has focused on the investigation of the neural correlates of tic generation, with particular attention to the brain mechanisms underlying the experience of the urge to tic. A better understanding of the sensory accompaniments of tics cannot only improve our understanding of the pathophysiology of tic disorders, but also inform clinicians about potential treatment avenues.
This paper reviews the existing literature on the clinical and neurobiological aspects of the premonitory urge in patients with TS, in order to present the state-of-the-art on current knowledge about its pathophysiology and possible treatment implications.
Clinical Phenomenology of Tics
Tics typically develop in childhood (average age at onset around 6 years; male to female ratio 4:1).
1,20 The most common tic at onset is eye blinking,
21 followed by other simple motor tics such as eye rolling, mouth opening, facial grimacing, shoulder shrugging, neck stretching, arm thrusting, kicking and abdominal contractions. Both complex motor tics (whole body movements, abnormal gait) and vocal tics (sniffing, grunting, throat clearing, humming, loud noises such as barking, as well as other vocalizations) are characterized by a later age at onset. Of note, out-of-context swearing as a complex vocal tic (coprolalia) is not included in the current diagnostic criteria as it is relatively rare, occurring in 10% of patients with TS in community settings and up to 30% of patients in specialist clinics.
22 Tic symptoms tend to follow a waxing and waning course throughout life, although most patients show improvement with time.
23,24 Environmental aspects, including social interactions, modulate tic severity,
25 and can trigger the expression of specific socially inappropriate behaviors.
26Large epidemiological studies and meta-analyses show that 0.3%−1% of school-age children fulfill diagnostic criteria for TS,
27,28 whereas tic symptoms are more common, affecting up to 50% of the general population at some point in life.
29 These figures are higher in children and in special education settings.
30 Tics characteristically present with a wide range of severity, from mild twitches that go unnoticed to forceful movements and loud noises that cause injury and call other people’s attention.
10 Moreover, 90% of patients present with comorbid psychiatric disorders,
31–33 most commonly obsessive-compulsive disorder (OCD)
34–36 and attention-deficit and hyperactivity disorder (ADHD),
37,38 although both affective disorders
39,40 and impulse control disorders
41–43 have also been shown to be associated with TS. Disease-specific health-related quality of life measures for patients with TS
44 have allowed a more accurate assessment of the differential impact of tics and behavioral symptoms on patients’ wellbeing.
45Clinical Phenomenology of the Premonitory Urge to Tic
Although urges to tic are not always easy to define, first-person anecdotal reports prompted further research into these core features of TS, which have been described as more bothersome than the tics themselves.
46 Of particular value are the subjective reports of patients with tics who published their accounts in early scientific articles. In 1980, Bliss provided the first detailed account of a series of “preliminary sensations” or “discrete sensations” that preceded or accompanied his motor and vocal tics.
47 He described sensory signals preceding his tics along with “
a very rapidly escalating desire to satisfy the sensations with movements intended to free oneself from the insistent feeling”
47. A few years later, Kane
46 expanded on this description by adding that “
these sensations are not mere precursors to tics […] they precipitate tics more than providing a signal of imminence, the pretic sensation acts as the aversive stimulus toward which tics are directed.” Indeed, Hollenbeck
48 acknowledged that some individuals perceive these premonitory urges and other sensory phenomena as being the “core” symptom of TS. Early accounts of subjective experiences associated with tic expression were captured by Shapiro et al.’s description of the clinical characteristics of what they termed “sensory tics”: somatic sensations in the joints, bones, muscles, and other parts of the body that trigger a feeling answered by performing an intentional or voluntary movement to relieve the disturbing sensations.
49–52The premonitory urge is commonly experienced in TS. In an early study of premonitory urges in 28 patients with TS aged 8–71 years, 82% reported premonitory urges prior to their tics.
53 Of note, 57% who reported premonitory urges found that these experiences were more bothersome than the tics themselves, and 55% thought the premonitory urges enhanced their ability to suppress tics. In a subsequent larger study of 135 patients with TS aged 8–71 years, 92% indicated that their tics were either fully or partially a voluntary response to their premonitory urges.
54 Consistent with Bliss’ account, 84% referred to unpleasant somatic phenomena that “build-up” prior to the tic (or upon attempts to resist ticcing) and are momentarily alleviated by tic expression.
54,55 Tics involving the head, neck, shoulders, or the midline abdomen tend to be most frequently preceded by urges, whereas simple tics, which are more brisk in nature, such as eye blinking and mouth movements, are less likely to be preceded by urges. With regard to anatomical location, premonitory urges are often described as being focal and limited to a specific body area, although in a minority these antecedent urges and sensations are more generalized and are best captured by a generic sense of inner tension (very rarely the urge can be extracorporeal, either in another person or in an inanimate object).
In 1994, Leckman et al. introduced the concept of “just-right” perceptions to describe the sensation that some patients with TS referred to as not feeling, looking or sounding well, balanced, or “just-right”
56. These distressing perceptions lead patients to perform the repetitive behaviors until they feel “just-right.” These authors reported that 44% of 130 patients with tic disorders aged 9 to 71 years reported “just-right” phenomena. Compared with other accounts of premonitory urges, these “just-right” perceptions were described as more of a mental phenomenon than a bodily sensation. The “just-right” phenomenon was most commonly related to visual (31%) or tactile (25%) sensory stimuli, as opposed to auditory (10%) perceptions. Moreover, these symptoms were significantly more common in patients with comorbid OCD (81%). This finding was replicated in a study by Miguel et al.,
57 who performed in-depth interviews with 21 adult patients with TS without OCD, 20 with TS and comorbid OCD, and 20 with OCD alone. The presence of at least one “just-right” perception was reported by 90% with a dual diagnosis, 48% with TS and just 35% of patients with OCD alone. The distribution of reported feelings of incompleteness followed the same pattern. A study by Worbe et al.
58 showed that 30% of 166 consecutive patients with TS aged 15–68 years endorsed the presence of “just-right” perceptions. Interestingly, patients who reported repetitive behaviors and thoughts that were “tic-like” (as opposed to “OCD-like”) had significantly higher rates of the “just-right” perceptions. In a recent study,
59 a standardized battery of self-report psychometric measures was administered to 71 adult patients with TS. Just-right experiences were systematically screened for using the Not Just Right Experiences-Questionnaire Revised (NJRE-Q-R), a self-report tool for the assessment of ten common just-right phenomena over the past month. The vast majority of patients in this clinical sample (80%) reported at least one just-right perception. Patients diagnosed with TS and comorbid obsessive-compulsive symptoms reported a significantly higher number of just-right experiences compared with TS patients without obsessive-compulsive symptoms. The strongest correlation was found between NJRE-Q-R scores and self-report measures of compulsivity, suggesting that just-right experiences might be intrinsic to the clinical phenomenology of patients with TS on a continuum with other sensory phenomena and can present with higher frequency in the context of comorbid tic-like compulsions.
Converging evidence suggests that patients first became aware of their premonitory urges on average 3 years after tic onset,
60,61 suggesting that premonitory urges may not be present during early stages of TS and emerge later. It has been proposed that with the development of premonitory urges, tics go through a twofold process of automatic negative reinforcement (the urges themselves) and positive reinforcement (the momentary relief from the urges following tic expression).
62,63 Kwak et al.
64 administered a questionnaire to 50 patients with TS (mean age 24 years) and found that 92% reported the presence of premonitory urges. Of these, 68% reported that their urges disappeared with tic expression. Banaschewski et al.
65 administered a similar questionnaire to 254 children with TS and documented the developmental trajectory of tic-related sensory phenomena: 24% of those aged 8–10 years, 34% of those aged 11–14, and 57% of those aged 15–19 reported the presence of premonitory urges. Interestingly, when these authors controlled for the fact that not all youth were able to report accurately whether or not they had an urge, the increasing trend in premonitory urge existence disappeared. Woods et al.
63 assessed premonitory urge phenomena in 42 children and adolescents with TS or a chronic tic disorder aged 8–16 years and found that 98% of patients reported the presence of premonitory urges. Premonitory urges are often difficult to describe in words, although some precocious young children are able to spontaneously assign names to their subjective experiences (e.g., “feeling tight,” “cramp”). It is rare that young children are able to report their awareness of premonitory urges before the age of 10 years.
64 It is likely that patients become aware of their urges to tic through a maturational process, which is largely independent of tic onset
63,66 and could be the expression of a transition from simple sensory perceptions to fully-fledged consciousness.
46,65Measurement of the Premonitory Urge
Two psychometric instruments have been developed to assess sensory phenomena associated with tics: the Premonitory Urges for Tics Scale (PUTS) and the University of São Paulo Sensory Phenomena Scale (USP-SPS). The PUTS is a self-report, unidimensional scale specifically designed to measure the severity of premonitory urges in patients with tic disorders.
63 This scale includes nine statements on premonitory urges that are rated using four generic anchor points (from 1=“not at all true” to 4=“very much true”). Total scores range from 9 to 36. Originally developed and validated in English, the PUTS has subsequently been translated and validated in other languages, namely German,
67 Hebrew,
66 and Italian.
68Overall, the PUTS has shown good psychometric properties, including convergent validity with scales rating the severity of tics and obsessive-compulsive symptoms, divergent validity with respect to ADHD severity, stability, and internal consistency. Specifically, PUTS scores showed good correlation with overall tic severity as measured by the YGTSS and the number, complexity, and interference YGTSS domains. Moreover, the results of a recent study by Brandt et al.
69 indicate that convergent validity between the PUTS and real-time urge assessment is good, and that the PUTS might assess more than one dimension of the premonitory urges (intensity and quality, as well as subjectively experienced control over tics and urges). However its clinimetric validity is poorer in subjects who are 10 years old or younger: in consideration of the absence of reliable biological markers, the assessment of premonitory urges relies on the age-related ability to report and describe complex sensory phenomena.
65 Developmental aspects could be involved in the child’s ability to recognize and accurately describe his/her somatic feelings, a meta-reflection process that requires the development of language and symbolization in order to represent the external and inner worlds.
70 The PUTS is therefore recommended for use only in patients older than 10 years, and its responsiveness to change is as yet uncertain.
71The USP-SPS was initially developed and validated in the Portuguese to evaluate sensory phenomena in Brazilian patients with tic disorders and OCD.
72 The USP-SPS consists of a checklist and a severity scale. The USP-SPS checklist covers examples of seven different types of sensory phenomena, and rates them as absent, previously present or currently present. If sensory phenomena are present, the patient is asked for the age of onset, plus any other subjective experience. The USP-SPS severity scale quantifies severity by rating frequency, associated distress, and interference with functioning on a 0–5 ordinal scale. The total score ranges from 0 to 15. Assessment of sensory hypersensitivity, which was not part of the original version of USP-SPS, is included in the English version of the instrument (checklist).
73 Specifically, subjects are considered to have sensory hypersensitivity if they rate themselves as more sensitive than other people to sensory stimuli, which they find overly distressing. This is relevant to patients with tics, as a clinical study showed that 80% of patients with TS reported heightened sensitivity to external stimuli, with examples among all five sensory modalities.
74 Of note, patients with TS do not demonstrate primary sensory deficits, suggesting that abnormal central sensorimotor processing and/or aberrant interoceptive awareness might underlie their clinically significant sensory abnormalities.
75 The USP-SPS has been shown to be psychometrically sound, with high sensitivity and acceptable specificity, suggesting its utility in assessing different sensory phenomena in patients of all ages. However, the English language validation study showed that sensory phenomena, obsessions, compulsions and tics were harder to differentiate in the pediatric sample (7- to 17-years old). A possible explanation for this could be that it is easier for a child to recognize if he/she had or not a described feeling than to quantify this feeling. Therefore, unlike the USP-SPS checklist, the USP-SPS severity scale is not recommended for use in the pediatric population.
Brain Correlates of the Premonitory Urge
Structural and functional neuroimaging studies exploring the neural correlates of the urge to tic have been conducted in the last decade, with the exception of an early study by Stern et al.
76 dating back to 2000 (see the
data supplement accompanying the online version of this article). This functional neuroimaging study using event-related positron emission tomography techniques combined with time-synchronized audio and videotaping of tics found that tic occurrence was highly correlated with increased activity in a set of neocortical (supplementary motor area, medial and lateral premotor cortices, dorsolateral-rostral prefrontal cortex, primary motor cortex, inferior parietal cortex, sensorimotor cortex, superior temporal gyrus, Broca’s area), paralimbic (insula, anterior cingulate cortex) and subcortical regions (claustrum, putamen, caudate nucleus). In the only patient with prominent coprolalia, the vocal tics were associated with increased activity within areas involved in mouth movements and speech production, including the prerolandic and postrolandic language regions, insula, caudate, thalamus, and cerebellum. In both simple tics and coprolalia, signal detection captured the neural signature of both urges to tic and actual tic expression. These preliminary findings were later replicated and refined by a further positron emission tomography study by Lerner et al.,
77 who reported activation of multiple cortical areas including the limbic (insula and anterior cingulate cortex) and supplementary motor areas in anticipation of tic expression.
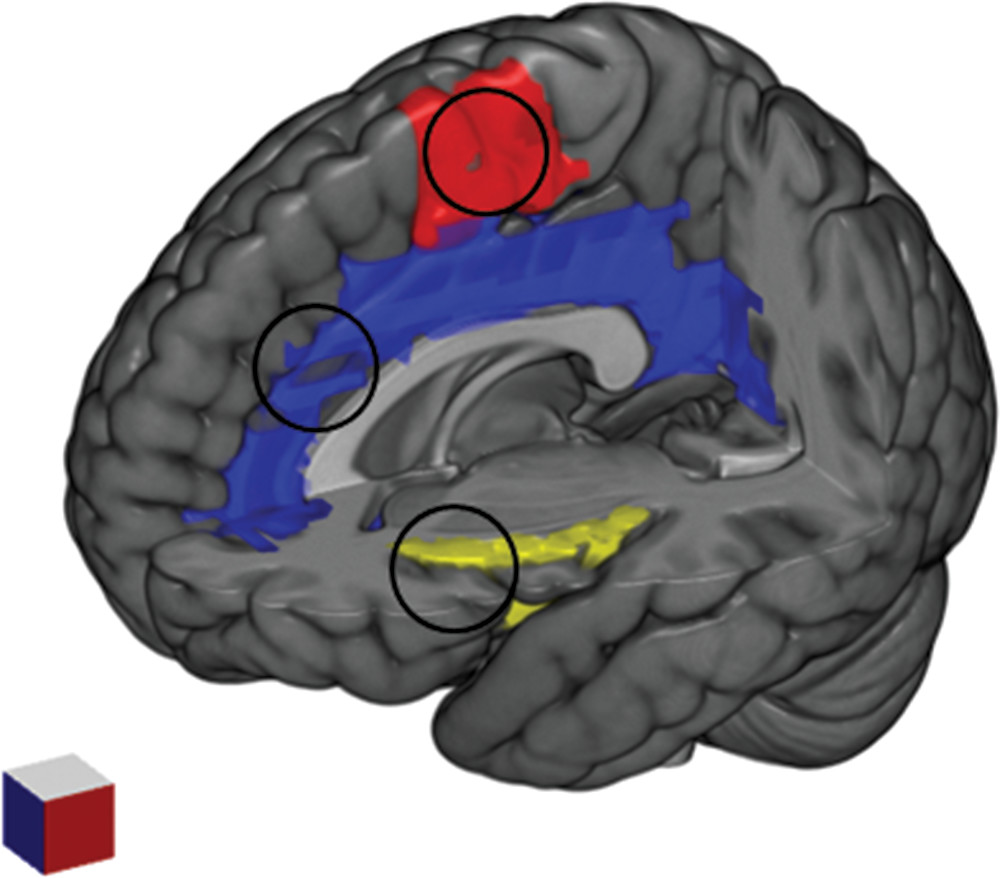
A number of studies using functional magnetic resonance imaging paradigms have provided more detailed information about the neurobiological signature of the urge to tic converging on insula, cingulate cortex and supplementary motor area (
Figure 1). Bohlhalter et al.
78 investigated the neural correlates of tics and associated urges using an event-related functional magnetic resonance imaging protocol. On the basis of synchronized video/audio recordings, activation patterns were analyzed two seconds before tic expression and at tic onset. A brain network of limbic areas, including the anterior cingulate and insular cortex, supplementary motor area, and parietal operculum, were activated prior to tic onset, concomitant with the subjective experience of the premonitory urge. This was followed at tic onset by activation in sensorimotor areas, including cerebellum and bilateral superior parietal lobule. The role of the supplementary motor area in tic generation was confirmed by Hampson et al.,
79 which compared brain activation patterns during tics and intentional movements. In this study, the supplementary motor area showed a significantly broader profile of cross-correlation to the motor cortex during tics than during matched intentional movements, performed by healthy control subjects. In addition to confirming the role of the supplementary motor area in tic generation, a subsequent study by Wang et al.
80 concluded that tics are caused by the combined effects of excessive activity in motor pathways (including the sensorimotor cortex, putamen, pallidum, and substantia nigra) and reduced activation in controlling regions of the cortico-striato-thalamo-cortical circuits (caudate nucleus and anterior cingulate cortex) [Wang et al.
80]. This study also found that activation in limbic regions, including the amygdala, can contribute substantially to tic generation. Neuner et al.
81 specifically addressed the distinction between the functional anatomy of tics at onset and the preceding activity that should be relevant for the urges to tic in a resting-state functional magnetic resonance imaging paradigm. The study revealed a specific temporal pattern for tic generation, with activation of the supplementary motor area, primary sensory cortex and parietal operculum occurring two seconds before tic onset; activation of a network involving the anterior cingulate cortex, putamen, insula, amygdala, cerebellum, and occipital cortex one second before tic expression; activation of the thalamus, central operculum, primary motor cortex, and somatosensory cortex at tic onset. Importantly, the findings of this study suggested that cortical activity preceded basal ganglia activity in the brain mechanism of tic generation. In line with these results, a more recent study by Tinaz et al.
82 reported that the functional connectivity between the right dorsal anterior insula and the bilateral supplementary motor area showed a positive correlation with both the urge to tic and tic severity in patients with TS.
The role played by sensorimotor processing in tic generation was confirmed by two recent magnetoencephalography studies: Biermann-Ruben et al.
83 showed that movement-evoked field amplitudes in a self-paced movement task were negatively correlated with both the severity and frequency of tics, suggesting that changes in sensory feedback loops during voluntary movements might also have an impact on tic control and that the sensory system and somatosensory-motor interaction are relevant to tic pathophysiology. Tinaz et al.
84 provided preliminary evidence that altered limbic input to the supplementary motor cortex, together with a change in the local supplementary motor cortex network function related to GABA activity, may contribute to a sensorimotor processing disturbance involved in tic generation.
The findings of structural neuroimaging studies also link alterations of paralimbic and sensorimotor regions and subjective reports of premonitory urges. Using a combined structural and spectroscopic magnetic resonance imaging, Puts et al.
85 showed that in vivo GABA concentration over the sensorimotor cortex of children with TS is reduced compared with healthy controls, possibly reflecting inhibitory dysfunction of both somatosensory and motor cortex. Finally, a recent study by Draper et al.
86 demonstrated that cortical thickness within the insular and sensorimotor cortex was inversely associated with ratings of the strength of premonitory urges in young adults with TS and that cortical thickness within the sensorimotor cortex, anterior cingulate, and insula was significantly reduced relative to a matched group of typically developing controls. These findings of significant cortical thinning within the sensorimotor, insular, and cingulate cortices of patients with TS confirmed similar findings reported by a previous morphometric magnetic resonance imaging study,
87 although it has been highlighted that the cortical thinning findings could also be explained by more frequent small-amplitude head movements during scanning in the TS groups.
88Pathological Urge to Tic and Physiological Urge for Action
It is a common observation that many of our everyday behaviors, including swallowing, coughing, yawning, and micturition, are characterized by bodily sensations that we experience as urges for action. These experiences share key subjective features with the premonitory urges associated with tics in TS and some repetitive behaviors reported in the context of other neuropsychiatric disorders (OCD, autism spectrum disorder, addictions). Jackson et al.
89 investigated the nature and functional anatomy of physiological and pathological urges for action in a quantitative meta-analysis of functional brain imaging studies, both in the context of everyday behaviors and in relation to clinical conditions. The authors’ review of previous theoretical frameworks about behavioral urges confirmed the presence of considerable phenomenological overlap between urges associated with everyday behaviors and urges associated with tic generation. Particular attention was paid to Davenport et al.’s motivation-for-action framework that had been proposed to account for the urge to cough.
90 The validity of this model, which stipulates that actions depend upon the conversion of an urge-for-action into a conscious desire-for-action, was called into question with reference with tic disorders. Specifically, it was highlighted that in the case of patients with TS urges-for-action are associated with the occurrence of motor and vocal tics that patients find both embarrassing and distressing. Moreover, the results of Jackson et al.’s
89 quantitative meta-analytic study using activation likelihood estimation demonstrated that the anterior insula and midcingulate cortex are the only regions of overlap across all brain imaging studies of behaviors driven by the perception of the urge for action. These two areas are also referred to as the limbic sensory and motor areas, respectively,
91 and are thought to play a key role in representing and experiencing the urge for action. Specifically, Jackson et al.
89 have proposed that these regions are part of a neural circuit that represents bodily sensations, generates urges for action, selects a particular action based upon a cost-benefit analysis of the likely “value” of that action (reward-based prediction analysis in cooperation with the ventral striatum), accumulates evidence on the outcomes of the selected action, determines whether the conditions giving rise to the urge have been resolved, and, if appropriate, generates a sense that the urge has been satisfied.
Lesion studies also provide convincing evidence that the insula may play a critical role in the experience of the urge for action. For example, a study by Naqvi et al.,
92 demonstrated that smokers who had sustained damage to the insula exhibit a disruption of their smoking addiction, described as “like their body forgetting the urge to smoke”. This hypothesis is in accordance with a recent neuroimaging study investigating the underlying physiology of spontaneous and voluntary eye blinking by analyzing event-related functional magnetic resonance imaging data in a block design involving blink suppression.
93 Results showed that activity in the bilateral insular cortex, right ventrolateral prefrontal cortex, and bilateral superior and middle temporal gyrus play a prominent role in the build-up of the physiological urge to blink. The authors highlighted that the insular cortex support a key role in the build-up of urge during blink suppression, consistent with prior findings on the involvement of the insular cortex in the perception of other bodily urges, thoughts, and behaviors. This observation suggests the existence of similar mechanisms across disorders associated with abnormal urge suppression, such as TS and OCD.
Jackson et al.
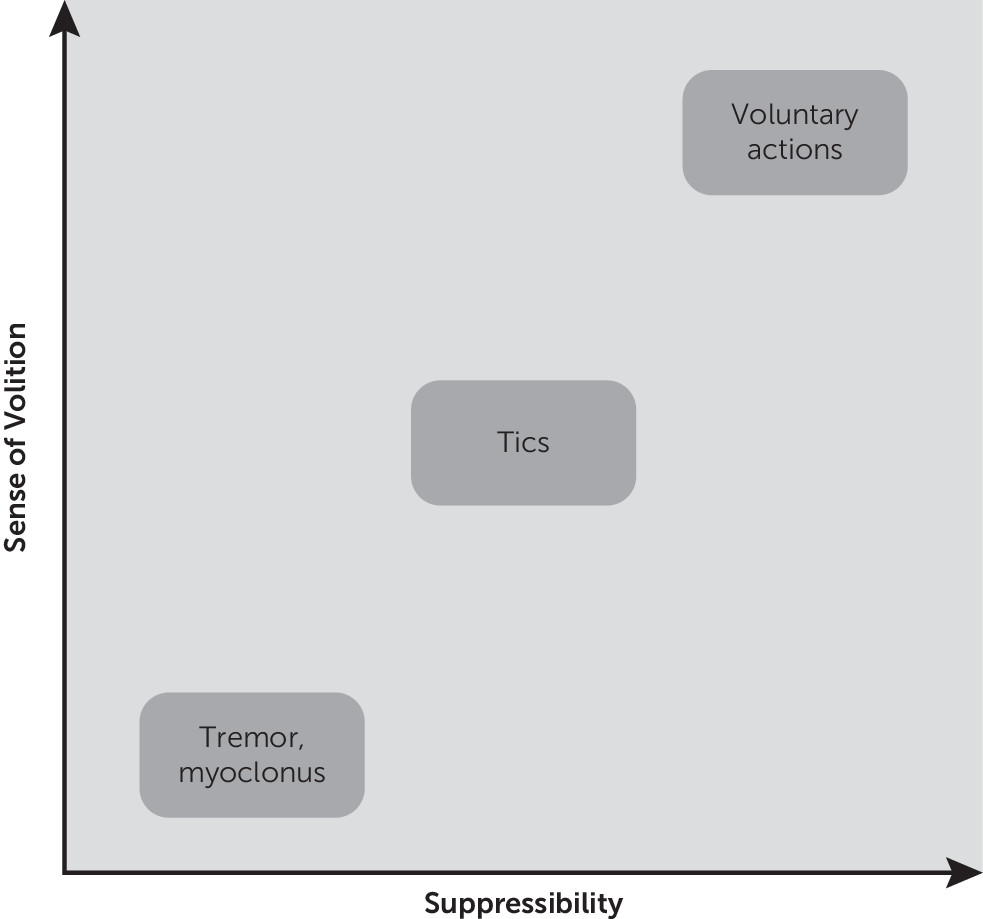
89 argued that this “motivation-for-action” network is anatomically separate and should be considered distinct from the neural system responsible for the preparation and execution of intentional, goal-directed, actions (“intentional action” network). The intentional action network is associated with regions of premotor and parietal cortex, which are likely to be responsible for the perception of “willed intention” (component of the sense of agency) during the execution of goal-directed actions. These neural models provide a useful framework for a naturalistic categorization of movements, which can be visually represented along the axes of sense of volition and suppressibility (
Figure 2). According to this framework, an action is voluntary when it is consciously performed, is flexible and can be controlled: the perceptual information is used to guide goal-oriented behavior. An action is involuntary when it is automatically performed, is inflexible but usually faster than a voluntary action: it cannot be controlled, because it is mechanically triggered by specific perceptual stimuli. Based on their heterogeneous features and presence of the urge, tics are often classed as a category of movement halfway between voluntary and involuntary behaviors.
94 Of note, studies on the neurophysiological correlates of movement disorders have yielded equivocal results, with an early study
95 showing that tics were not always preceded by the readiness potential (Bereitschaftspotential), and a subsequent study
96 showing that tic generation was associated with the readiness potential in 2 out of 5 patients. It has been proposed that tics associated with a premonitory urge (and therefore consciously perceived and voluntarily initiated) are more likely to be preceded by the readiness potential, as shown in three patients in the only study to date that addressed this question
97 and in a more recent study by Moretto et al.
98 demonstrating that patients with TS have a delayed experience of volition. Van der Salm et al.
99 found that patients with psychogenic jerks have a readiness potential prior to their movements significantly more often and with a significantly earlier onset than patients with TS. Finally, although tics are considerably less frequent during sleep, they have been observed during all phases of sleep, including slow wave sleep,
100 suggesting that at least some tics are involuntary.
From a theoretical perspective, further work needs to be done to elucidate the multifaceted relationship between the concepts of reflex, urge, and intention. Any useful model for the understanding of premonitory urges/urges for action should be integrated with more general models on the neural correlates of consciousness and its neural correlates.
94 More specifically, it would be important to address the question whether it is the strength of activation of the insular cortex, the cingulate cortex, or the circuit between these regions that is responsible for setting the threshold between unconscious and conscious awareness of urges to tic in patients with TS. The relationship between the perception of the premonitory urge and effortful tic suppression also deserves further investigation, based on Jackson et al.’s
89 observation that an urge to act is, at least in the majority of instances, an awareness of the effort involved in restraining the act. According to this view, it would be conceivable for sensory inputs to trigger actions out of awareness (involuntary actions, without urges), however if the actions are delayed, the sensations reach awareness and urges to act (premonitory urges to tic) develop.
Diagnostic and Treatment Implications
Different degrees of awareness and intentional control accompany human actions, ranging from goal-directed behaviors to automatic habits or behavioral routines
94 (
Figure 2). Sensory phenomena are frequently reported in association with tic expression, and are currently recognized as core symptoms of TS, having been incorporated in clinical rating instruments such as the Diagnostic Confidence Index for TS.
101 Recommendations from the European Society for the Study of Tourette Syndrome state that formal assessment of sensory phenomena should be included as part of the standard clinical evaluation of patients with TS and other tic disorders.
102 Recent studies have highlighted the role of the clinical assessment of premonitory urges as hallmark features of TS for the differential diagnosis of various hyperkinetic jerky movements, including psychogenic jerks.
103 Moreover, the presence of premonitory urges, along with tic severity and family history of TS, has been identified as an important predictor during childhood of a poorer health-related quality of life in adults with TS.
45 Increased insight into their subjective experiences could help patients to better recognize exacerbating and alleviating factors for their tic symptoms and ultimately improve their ability to suppress them. In fact, Himle et al.
104 investigated the effects of tic suppression on premonitory urge ratings and found that some children reported higher urge ratings during periods of tic suppression. Arguably the most promising preliminary finding related to tic suppression is that a behavioral intervention consisting of exposure to premonitory sensory experiences during prolonged tic suppression may be beneficial in the treatment of tics.
105 In general, an increased awareness of premonitory urges has promoted the development of various cognitive behavioral interventions for tics. A multicomponent Comprehensive Behavioral Intervention for Tics has shown good effectiveness in enhancing the patient’s awareness of the premonitory urges and teaching them how to perform a competing behavior (habit reversal training) whenever they sense their tics are about to occur.
106–108 Overall, there are few studies evaluating the association between sensory phenomena and treatment response. Nevertheless, it is possible to hypothesize that these sensory phenomena may be useful predictors of treatment response. For example, the severity of premonitory urges has been shown to improve as the tics improve with some treatment interventions beyond dopamine blockade, including topiramate and botulinum toxin.
109,110Although the development of specific instruments such as the PUTS and the USP-SPS has made it possible to better characterize and monitor changes in the intensity of premonitory urges, there is still a paucity of studies using structured interviews to assess the presence and severity of sensory phenomena, as well as investigating the associated epidemiology and etiological mechanisms. Specifically, it would be important to reach a consensus on a definition of tic-related subjective experiences that encompasses all previous descriptions of sensory phenomena in patients with TS and other tic disorders. Likewise, further research on sensory phenomena could be useful to identify more homogenous clinical phenotypes of TS, as well as OCD and impulse control disorders, thus forming the basis of more sophisticated genetic correlation studies. From the neurobiological point of view, elucidating the exact nature of the relationship between activation of motor (supplementary motor area) and paralimbic (insula and midcingulate cortex) brain regions would provide crucial insights into the pathophysiology of the urge to tic. A better understanding of the neural correlates of sensory phenomena could yield valid and reliable indicators of the pathophysiology, prognosis, and treatment response in patients with TS. Further research is needed to address the abnormal mechanisms linked to the phenomenon of “somatic hypersensitivity” or “site sensitization,” which seems to be supported by instances of sensory hypersensitivity frequently reported by patients with TS.
74 The possibility that patients with focal premonitory urges show a preferential lateralized activation of the relevant networks on neuroimaging
82 should be tested in future studies. Finally, the delayed appearance of premonitory urges raises the question whether the association of urges and tics is a compensatory phenomenon that forms the basis for the capacity to suppress these symptoms, or whether urges and tic suppressibility are coexisting phenomena, as suggested by recent tic-suppression studies
111,112. As shown by this review article, sensory experiences are of considerable theoretical and clinical importance in understanding and treating TS and other tic disorders, and yet their precise role in the occurrence of tics, and their exact relationship to tics, remain the subject of fruitful debate within both consciousness studies and neuropsychiatry.