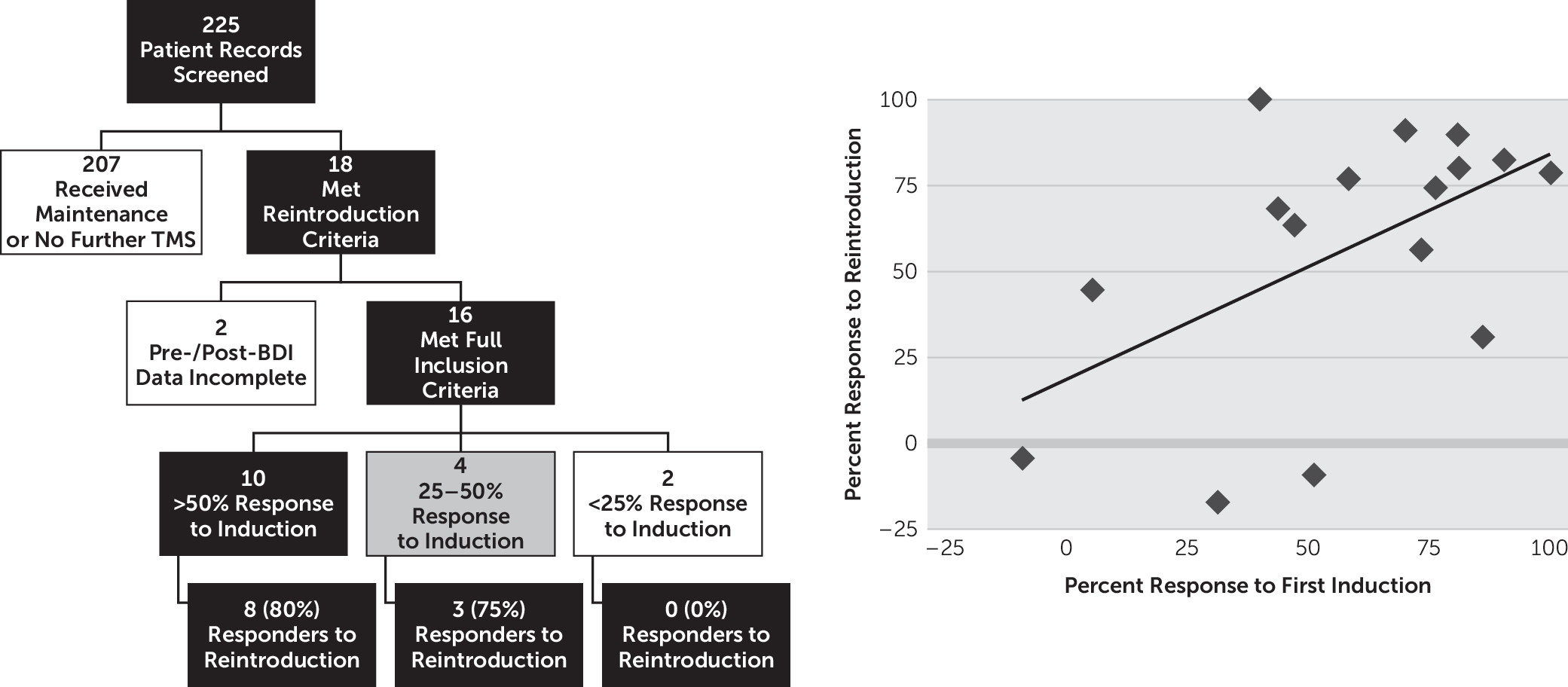
The results presented above support the hypothesis that therapeutic response to an initial course of rTMS for depression is a significant predictor of response to a subsequent course. This finding is consistent with other literature on treatment response, with several authors reporting high response rates to reintroduction in patients who had responded initially.
2,4,9,11 Our work, showing that the magnitude of initial antidepressant explains approximately one-third of the magnitude of response to reintroduction, has implications for management of depressed patients with a past favorable response to rTMS. We provide support for an approach involving watchful waiting and reintroduction of TMS when such patients experience a relapse, thus placing rTMS as a viable long-term treatment regimen for treatment-resistant depression. There is also an indication that those who do not respond (BDI change <25%) to initial induction may be less likely to respond to further treatment, but this topic warrants evaluation in larger samples of nonresponders.
The current findings are consistent with the existing literature on the topic of reintroduction. In 2000, Dannon et al.
13 reported a case series of four patients who responded to a course of reintroduction to a similar degree relative to the induction course. In 2006, Fitzgerald et al.
14 followed 19 responders who received reintroduction, showing that 12 responded (63%) and 17 of 19 had at least a near response (25% improvement). Two years later, Demirtas-Tatlidede et al.
11 showed, in a case series of 14 patients, that treatment response to rTMS was reproducible after relapse, with patients receiving reintroduction on average every 5 months. Janicak et al.
4 showed, in 2010, that within the first 6 months after induction treatment, 38 of 99 patients experienced symptom worsening, with 32 of 36 (84%) benefitting from reintroduction of TMS. More recently, in a large naturalistic observational study lasting 12 months, Dunner et al.
2 showed that, among 98 patients receiving reintroduction in the setting of symptom worsening, those with the best response to induction were the least likely to experience a relapse after reintroduction. Our findings build on the literature summarized above, showing that the best indicator of response to reintroduction is response to induction. Importantly, these findings also give both patients and physicians an understanding that magnitude of response to reintroduction will likely be similar to the initial response.
Patients treated with rTMS tend to have severe, refractory depression. Thus, converting initial therapeutic response into successful long-term management represents an important and still under-investigated topic for the field. Currently, there is no clear consensus on how this should best be accomplished. These findings, along with the literature reviewed above, contribute to this ongoing discussion. Limitations of the study include a relatively small and heterogeneous sample identified retrospectively via chart review. Moreover, though initial response was found to be a significant predictor of reintroduction response, it only accounts for 29% of the variance in scores. Thus, other factors, either not evaluated in this study or requiring a larger sample size to achieve significance, such as gender, age, refractoriness, baseline severity, and comorbid disorders, could also have a significant predictive role.
15 However, the current study results reflect the experience of a clinical program and thus have practical relevance. Furthermore, we hope these findings may spur additional prospective research, ideally comparing long-term rTMS treatment strategies, including maintenance rTMS and a combination of watchful waiting and reintroduction therapy upon symptomatic relapse.