Among the many pain syndromes afflicting humans, back pain is the most common, with a lifetime prevalence approaching 80%, and chronic back pain often results in disability, opioid dependence, and high health care costs.
1 Stroke is also common, and whereas data are limited, cerebral infarcts may lead to resolution of chronic pain. Such cases are exceedingly rare, and although stroke relieving thalamic pain
2 and phantom limb pain
3 has been observed, relief from chronic back pain after stroke has not, to our knowledge, been reported.
Case Report
A right-handed man developed back pain at age 21 when he injured his lower back during military service. He gradually developed chronic lower back pain and had lumbar back surgery at age 31 and at age 32, with little relief. He then sustained additional back injury in a motor vehicle accident at age 46, leading to lumbar laminectomy. The patient’s back pain was not relieved, and after another lumbar laminectomy at age 49, he became unemployed from his job as a steel mill worker because of chronic pain from failed back syndrome. The pain was primarily in the lumbar region and was described as dull, persistent, and nonradiating. After his last back surgery, the patient was continuously administered opiate medications in an attempt to control the back pain. His peak opiate doses were methadone 20 mg t.i.d. and acetaminophen/hydrocodone 500 mg/5 mg 120–180 times per month, and his optimal response to these medications was the endorsement of 5 on a 10-point pain rating scale.
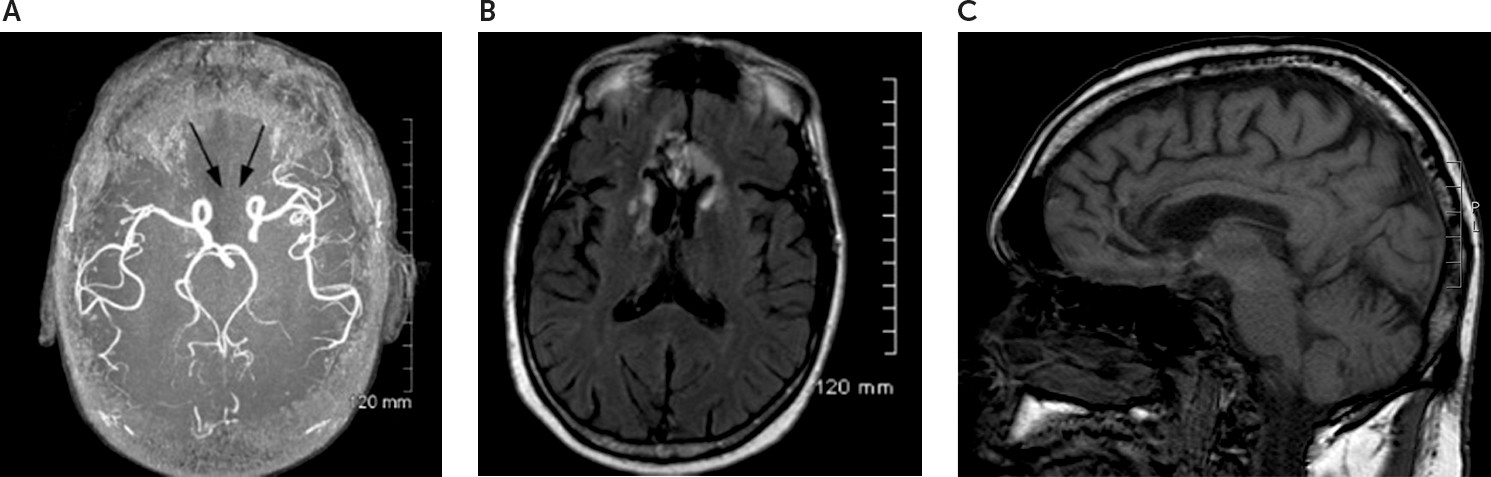
At age 59, he was found unconscious and taken to an emergency department where he was somnolent and disoriented. Examination was limited by his somnolence but disclosed normal cranial nerves 2–12, symmetric reflexes, and no focal deficits. The patient’s urinary toxicology screen was negative, and his carboxyhemoglobin level was normal. Stroke in the distribution of the anterior cerebral arteries (ACAs) was identified by neuroimaging (see
Figure 1). Three days later, neurologic consultation was obtained because of cognitive dysfunction. The patient was found to be alert but disoriented and abulic, with normal speech and language, as well as symmetric reflexes with flexor plantar responses. Carotid ultrasound, echocardiogram, complete metabolic panel, complete blood count, urinalysis, vitamin B
12 levels, thyroid stimulating hormone, hemoglobin A1C, erythrocyte sedimentation rate, and C-reactive protein were unremarkable. CSF analysis showed a glucose level of 61 mg/dl; protein level of 79 mg/dl; 65 white blood cells (92% lymphocytes, 8% monocytes); 468 red blood cells; negative bacterial, fungal, and viral cultures; nonreactive Venereal Disease Research Laboratory test; and no oligoclonal bands. Four weeks after admission, the patients was oriented only to his name, and slight weakness of the lower extremities was apparent. He had a history of hypertension, hyperlipidemia, chronic obstructive pulmonary disease, posttraumatic stress disorder, depression, and, notably, a right cerebral white matter lacune at age 55 that had no effect on his chronic lower back pain. After his evaluation, he was sent to a rehabilitation facility where he slowly improved.
Ten weeks after his stroke, the patient had no complaints of lower back pain and was taking no pain medication. On examination, he was awake and alert but disengaged and apathetic. Retrospective assessment using the Apathy Scale,
4 conducted by C.M.F. and C.A.A., disclosed a score of 33/42, consistent with severe apathy. The patient’s attention, language, praxis, gnosis, and visuospatial function were normal, but perseveration was noted on clock drawing. His memory was impaired, with 0/3 recall at 5 minutes. His Mini-Mental State Examination
5 score was 21/30 (Z score=−3.18).
6 Cranial nerves II–XII, strength, bulk, tone, coordination, sensation, and reflexes were normal. His gait was mildly unsteady, and use of a cane was necessary. His plantar responses were flexor, but snout and grasp reflexes were present.
During subsequent observation of the patient, lasting 2 years and 3 months, cognitive dysfunction persisted, with disinhibition (i.e., masturbating in public) alternating with apathy, impaired judgment, and violent mood swings, and he had urinary incontinence, leading to total dependence on his family for daily care. However, he never needed or requested medication for his back pain. At age 61, he replied “0” when asked to rate his pain level on a scale of 0–10. Soon thereafter, he was admitted to a nursing home facility because his family could no longer care for him, and he was lost to follow-up.
Magnetic resonance angiography (MRA) and magnetic resonance imaging (MRI) were obtained 3 months after stroke onset. MRA disclosed bilateral occlusion of the ACA (
Figure 1A). MRI disclosed bilateral involvement of the anterior cingulate gyrus (portions of Brodmann areas 24, 25, 32, and 33), the gyrus rectus (portions of areas 11 and 12), the nucleus accumbens, the caudate head (
Figure 1B), and the genu of the corpus callosum (
Figure 1C). The prior lacune in the right subcortical white matter was also present.
Discussion
The patient in the above case had refractory chronic lower back pain for more than 10 years requiring large doses of opiates. However, after bilateral ACA distribution stroke, he neither needed nor sought drugs of this class or any analgesia during follow-up of more than 2 years. Given the location of the infarction, involving components of structures critical for pain perception in both cerebral hemispheres and their callosal connections, we conclude that the cerebral damage produced complete and lasting resolution of chronic back pain. The observation that a prior stroke in the right subcortical white matter had no effect on pain provides further evidence for the specific effect of this particular infarct on pain resolution.
Pain perception has been investigated for decades,
7,8 but in recent years functional neuroimaging studies have suggested that pain activates a variety of brain regions corresponding to three major dimensions of pain: sensory, affective, and cognitive.
7,9,10 Crucial areas identified include the thalamus and somatosensory cortices for sensory processing (the encoding of information about pain features such as location and duration); limbic structures, including the anterior cingulate gyrus, nucleus accumbens, amygdala, and insula for affective processing (the emotional experience of pain); and the dorsolateral prefrontal cortex, parietotemporal cortices, and periaqueductal gray for cognitive processing (the direction of attention either toward or away from pain).
9,10 White matter connections between these regions are also increasingly recognized as important elements of these networks.
11Most important for those suffering from chronic pain is the affective dimension and the structures implicated in affective processing—all components of the limbic system–are those that sustained major damage in the above case. A central role of the limbic system in the experience of pain has long been recognized. Indeed, the limbic system was conceptualized as critically involved in emotion by James Papez in 1937.
12 In particular, the anterior cingulate gyrus was thought by Papez to be “the seat of dynamic vigilance by which environmental experiences are endowed with an emotional consciousness”.
12 Moreover, the nucleus accumbens, which receives dopaminergic neurons from the ventral tegmental area and sends projections to the anterior cingulate gyrus, is increasingly recognized to play an important role in reward-based learning and opioid analgesia,
9 implying that the integrity of this region is essential for the therapeutic action of opiates.
The novel observation of the present case was enabled by an unusual stroke syndrome. Stroke in the distribution of the ACA is uncommon, and bilateral involvement is extremely rare, accounting for 0.1% of all ischemic cerebral infarcts.
13 Whereas infarction such as that seen in our patient often follows ACA aneurysmal rupture, our patient did not present with headache, the CSF profile was inconsistent with subarachnoid hemorrhage, and the neuroimaging findings were most suggestive of bilateral ACA occlusion.
Our patient’s lesion, involving the anterior cingulate gyri and both nuclei accumbens, and their callosal connections, can plausibly be interpreted as being responsible for abolishing the experience of pain, producing the disappearance of opiate-related reward, or a combination of both. The prominence of apathy and abulia is consistent with either interpretation, as these features are typical of bilateral anterior cingulate gyrus damage and can be expected with the ablation of brain reward systems. Lesions in these structures, particularly Brodmann areas 24
8 and 25
14 of the anterior cingulate gyrus, may be usefully conceptualized as disrupting ventral limbic structures supporting emotional generation, expression, and experience, with the result that affected individuals do not endorse pain. Whereas we suspect that our patient no longer had the experience of pain after his stroke, it is possible that pain was still present because intact sensory regions maintained the peripheral generation of pain from unresolved lumbar spine disease, and he was simply unconcerned about it so that he no longer suffered. We cannot answer this question; however, future investigation may help clarify this issue. As a detailed clinical observation, this case offers useful insight into the central organization of pain perception, most importantly the affective component of pain that is so often associated with profound suffering.