Pseudobulbar affect (PBA) is considered a syndrome of affective disturbance characterized by frequent, brief episodes of inappropriate and functionally impairing laughter and crying to a minor stimulus, independent of an individual’s mood.
1 Affect is defined as a “pattern of observable behaviors that is the expression of [transient] subjectively experienced feelings” of various emotional states, whereas mood is a “pervasive and sustained emotion” that may last for several days or weeks.
2 Disorders of affect such as PBA are distinct syndromes found in many neurological diseases and acquired brain injury and are associated with a high burden of illness.
3,4 Additionally, sometimes referred to as “pathological laughing and crying” or “affective lability,” PBA gained recognition in the 1960s in patients with bulbar variants of amyotrophic lateral sclerosis (ALS), a disorder of progressive degeneration of upper and lower motor neurons.
5 PBA and its associated therapies have been primarily studied in ALS and multiple sclerosis, a demyelinating disorder of the central nervous system.
6,7 However, more recently PBA has been described in patients with movement disorders such as Parkinson’s disease (PD) and atypical parkinsonism (aP), characterized by degeneration of basal ganglia, brainstem, and cerebellar neurons.
3,8,9 The pathophysiology of PBA remains unclear; proposed mechanisms include disruption of voluntary motor control over brainstem reflexes (i.e., cortical disinhibition) and direct impairment of brainstem responses to sensory stimuli, resulting in an exaggerated and inappropriate reaction.
10,11 While the presence of PBA is believed to be independent of mood disturbance
12, Strowd and colleagues
9 reported an association with PBA and depressive symptoms in patients with various movement disorders, including PD, though comparable studies have not been performed in ALS populations.
Despite the differences in clinical phenotypes and pathophysiology in disorders associated with PBA, to our knowledge there are no studies that have systematically studied neuropsychiatric correlates of PBA in different neurological disorders. Furthermore, cortical disinhibition of primitive reflexes (i.e., frontal release signs) is frequently identified in dementia syndromes that may concurrently develop in patients with ALS, PD, and other movement disorders.
13–15 Yet the association between PBA and cognition has not been defined. In this pilot study, we aim to describe the neuropsychiatric correlates of self-reported PBA symptom severity in patients with PD, atypical parkinsonism, and ALS.
Methods
This exploratory cross-sectional study was approved by the Baylor College of Medicine Institutional Review Board. A total of 108 patients (PD, N=53; aP, N=29; ALS, N=26) were recruited from a subspecialty focused tertiary care center between January 2012 and February 2013. All patients were diagnosed as having clinically definite PD or aP by a movement disorder neurologist according to accepted clinical criteria. The aP group consisted of eight patients with progressive supranuclear palsy, 11 patients with multiple system atrophy (MSA), and 10 patients with corticobasal degeneration. ALS was diagnosed according to accepted clinical criteria by a neuromuscular specialist. Patient group sizes were recruited with consideration of population-based disease prevalence. Participating subjects completed the following series of self-report questionnaires: the Center for Neurological Study–Lability Scale for pseudobulbar affect (CNS–LS); the Beck Depression Inventory, 2nd edition (BDI–II); the State-Trait Anxiety Inventory (STAI, trait only); the Apathy Evaluation Scale (AES–Self- and Other Report); the 36-Item Short Form Survey (SF–36), a quality of life measure; and the Montreal Cognitive Assessment (MoCA). (Individual MoCA total scores were standardized to Z scores and corrected for age and education by using normative data by Rossetti and colleagues.
16) Patients were excluded if they did not speak English, if they were unable to communicate answers due to advanced disease, or if they did not complete >30% of the questions. Group assignment was based on whether or not the participant had a total score ≥13 on the CNS–LS (PBA[+] or PBA[–], respectively, in which PBA[+] suggests the presence of symptoms compatible with the diagnosis of PBA.
17)
All statistical procedures were run in SPSS (version 23.0, IBM, Armonk, N.Y.). Overall group differences on outcome variables were evaluated by using one-way analysis of variance (ANOVA) tests with Tukey’s Honestly Significant Difference Test follow-up contrasts. Two-way analyses of covariance were used to investigate possible interactions for PBA(+) and clinical group (PD, ALS, aP) on outcome variables. Effect sizes are presented in Hedges’s g. Pearson’s product moment correlations were calculated to examine the relationships between CNS–LS scores and various outcome variables. Multiple linear regression was used to investigate whether related clinical correlates predicted self-reported PBA symptom severity. Demographic variables were analyzed with chi-square tests of difference and t tests. Alpha was set at 0.05, two-tailed, for all inferential tests.
Results
Patient Characteristics
Descriptive statistics revealed that all continuous variables were normally distributed. Examination of skewness statistics, scatterplots, and boxplots revealed no problematic outliers or significant skewness for any variables. Demographic and other clinical characteristics of the final study groups are presented in
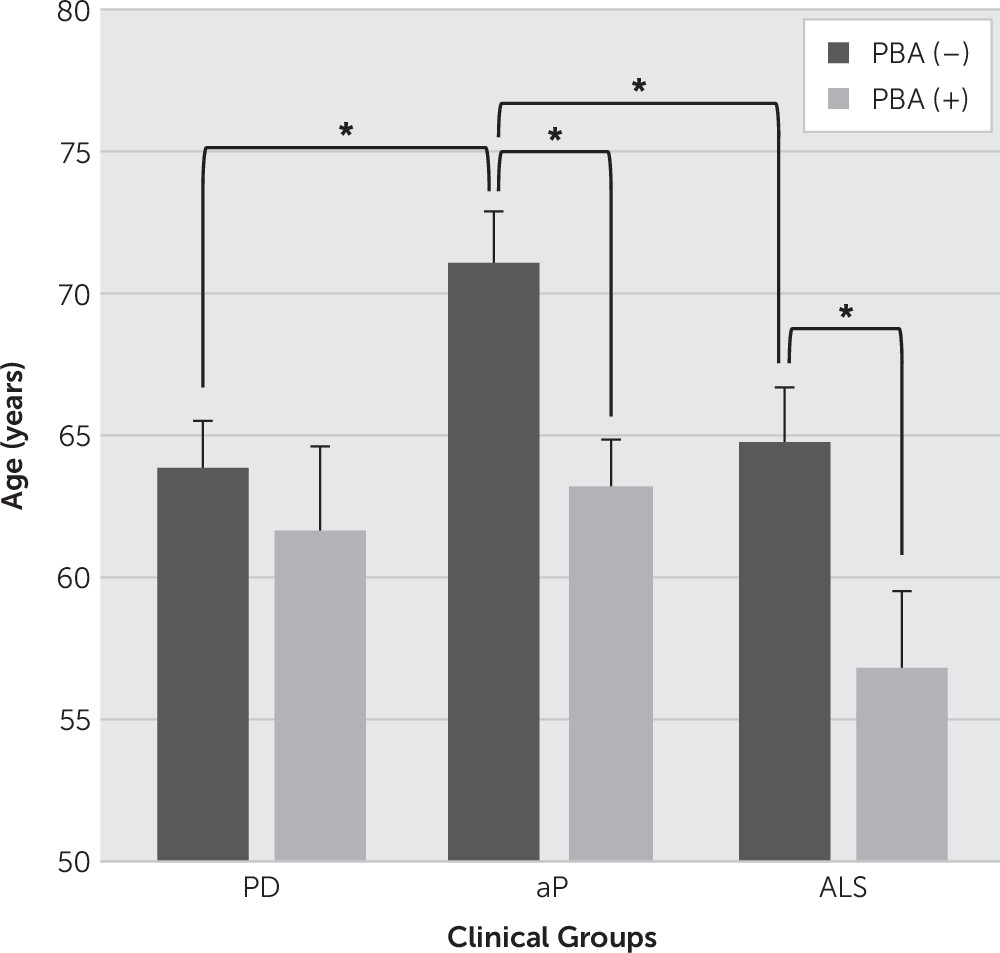
Table 1. The clinical groups did not differ significantly in terms of gender, education, ethnicity, or duration of illness. There were significant group differences regarding age at onset of neurologic disorder (F=3.80, df=2, 107, p=0.03) and for current age (F=3.31, df=2, 107, p=0.04). Patients in the aP group were significantly older than those in the ALS group at the time of testing and were diagnosed with a movement disorder later in life than patients in the PD group. This is consistent with the clinical phenotypes of these diseases and potential for delayed diagnosis in aP. Both current age and age at onset were significantly related to scores on the CNS–LS, AES–Self-Report, and AES–Other Report (data not shown), such that younger age and younger age at onset were associated with higher (i.e., less optimal) scores on each measure. Furthermore, a 3 (clinical group)×2 [PBA(+) or (–)]) ANOVA revealed significant main effects for presence of PBA symptoms (CNS–LS score ≥13) and clinical group. Specifically, PBA(+) patients were significantly younger than PBA(–) patients in the aP and ALS groups, but not in the PD group (see
Figure 1).
Group Differences in PBA
While this study was not designed to determine the prevalence of PBA, the distribution of PBA(+) individuals in our cohort was 37.9% for aP patients (N=11), 20.8% for PD patients (N=11), and 34.6% for ALS patients (N=9). These rates are consistent with what has been demonstrated previously. There were no statistically significant differences in proportion of PBA(+) individuals within each clinical group (PD: χ2=3.287, p=0.193). Additionally, there were no statistically significant differences in proportion of individuals for varying CNS–LS cut-scores (≥13, ≥18, and ≥21; p=0.57) between clinical groups. There were also no significant differences in mean CNS–LS scores between clinical groups (PD: 10.11±3.83; aP: 11.66±5.23; ALS: 11.42±6.92 [F=1.32, df=2, 107, p=0.27]). Subscore analyses of CNS–LS laughter and crying items revealed no significant overall group differences between clinical groups (CNS–LS laughing: F=2.47, df=2, 105, p=0.09; CNS–LS crying: F=0.64, df=2, 107, p=0.53). However, patients in the ALS group endorsed more laughing symptoms than those in the PD group (t=–2.21, df=75, p=0.03, g=0.54).
Group Differences on Outcome Variables
Results from a 3 (clinical group)×2 [PBA(+) or (–)] ANOVA for various outcome variables revealed significant main effects for clinical group on informant report of the AES (F=3.50, df=2, 108, p=0.03) and the following subscales of the SF–36: physical functioning (F=8.53, df=2, 108, p<0.001), role limitations due to physical health (F=7.81, df=2, 108, p=0.001), and general health perceptions (F=6.74, df=2, 107, p=0.002). Significant main effects for clinical group and cognition were found for total MoCA score (F=3.53, df=2, 108, p=0.03); however, these effects were no longer significant when corrected for age and education. Follow-up Tukey’s Honestly Significant Difference Test (see Table S1 in the
data supplement accompanying the online version of this article) revealed that the aP group was significantly less apathetic than the PD group (t=6.37, df=80, p=0.04, g=0.98). Both aP and ALS patients had significantly lower scores than PD patients on the SF–36 physical functioning, role limitations due to physical health, and general health perceptions subscales. It is noteworthy that there were no differences between diagnostic groups on the BDI–II, STAI, AES–Self-Report, and the SF–36 subscales for bodily pain, vitality, role limitations due to emotional problems, and mental health (
Table 2).
Among all subjects, significant main effects were noted for PBA(+) group assignment on the STAI (F=8.06, df=1, 107, p=0.005), as well as a marginally significant effect on the BDI–II (F=3.50, df=1, 107, p=0.06), indicating that PBA(+) patients had greater levels of anxious and depressive symptoms (
Table 3). There were also significant main effects for PBA(+) group assignment on the following SF–36 subscales: social functioning (F=7.46, df=1, 107, p=0.01), role limitations due to emotional problems (F=4.46, df=1, 108, p=0.04), and mental health (F=6.18, df=1, 108, p=0.02), suggesting that a CNS–LS score ≥13 is associated with poorer quality of life. There were no significant interactions between clinical group and PBA(+) group assignment among any outcome variables (data not shown).
Relationship Between PBA Symptoms and Outcome Variables
A Pearson product moment correlation matrix, including data from all participants, revealed that PBA symptom severity (CNS–LS total score) was significantly related to BDI–II (r=0.27, p=0.004), STAI (r=0.38, p<0.001), SF–36 social functioning (r=–0.28, p=0.004), SF–36 emotional role functioning (r=–0.20, p=0.04), and SF–36 mental health (r=–0.35, p<0.001), once again associating PBA symptoms to depression, anxiety, and poorer quality of life. Given the significant overlap between PBA crying symptoms and depression, partial correlations were calculated to determine the relationship between PBA symptom severity and various outcome variables controlling for depressive symptoms. After controlling for depression, PBA symptom severity continued to be significantly related to STAI (r=0.34, p=0.001) and SF–36 mental health (r=–0.26, p=0.02). MoCA scores did not correlate with PBA symptom severity. Pearson product moment correlation matrices separated by clinical group revealed CNS–LS score was significantly related to BDI–II, STAI, anxiety, and mental health quality of life for PD and trending toward significance for aP patients. Of note, CNS–LS score was significantly related to age and education and corrected MoCA scores for participants in the aP group but not for the other clinical groups. No significant relationships were demonstrated in the ALS group (
Table 4).
Multiple regression analysis was used to test if noteworthy clinical correlates (i.e., STAI, BDI–II, age) significantly predicted a patient’s CNS–LS score, to investigate whether a regression equation to predict a patient’s score on the CNS–LS could be devised. On the first step, age at testing was entered into the model and was significantly correlated with CNS–LS score (R2=0.07, F=8.02, df=1, 104, p=0.01). On the second step, BDI–II, AES–Other Report, and STAI scores were entered simultaneously, resulting in a significant increase in full model R2 (F=8.01, df=4, 86, p<0.001, R2=0.28). Within the full model, anxiety scores (STAI) significantly predicted PBA severity above and beyond the variance explained by age (β=0.17, p=0.003). The variance explained by anxiety fully accounted for the relationship between BDI–II score and AES–Other Report score to CNS–LS score, such that depression and apathy were no longer predictive of PBA symptom severity when accounting for anxiety (βBDI-II=0.12, p=0.06; βAES-O=0.05, p=0.34).
Discussion
Despite the presence of PBA in a variety of neurological disorders, little is known about its underlying mechanisms. To better define this syndrome, we chose to compare the clinical correlates of PBA symptoms in disorders with distinctly different pathophysiology. To our knowledge, this pilot study is the first to systematically identify the neuropsychiatric correlates of PBA symptoms between different neurological disorders.
In this study, PBA(+) patients were typically younger than PBA(–) patients, with comparable rates (approximately 30%) and severity of symptoms between groups. Though there was a trend for ALS patients to endorse more inappropriate laughter than PD patients, generally speaking, the clinical groups did not differ in terms of PBA symptom presentation. Despite the impact of the underlying illness on the patients’ physical and cognitive well-being, PBA(+) patients exhibited greater levels of depressive and anxious symptoms, but not apathy, and decreased mental health quality of life in all groups. Furthermore, age and severity of anxiety accounted for most of the variance in whether a patient exhibited significant PBA symptoms. Younger patients with high levels of anxiety were most likely to exhibit symptoms compatible with a diagnosis of PBA, regardless of the patient’s neurological condition.
Another novel finding is that PBA(+) status correlated with presence of mood-related symptoms (i.e., depression, anxiety, apathy, low mental health quality of life) in PD and aP patients but not in ALS patients. This corroborates previous findings of an association between depression and PBA in a movement disorders population.
9 Our study also emphasizes the contribution of a patient’s level of anxiety in predicting the presence of PBA in patients with parkinsonism (including PD and aP). In these groups of patients, it is therefore suggested that mood symptoms should also be assessed when PBA symptoms are identified and vice versa, in order to more appropriately guide therapeutic decisions. Although PBA is considered an affective disorder independent of mood, both antidepressants (such as serotonin reuptake inhibitors and tricyclic antidepressants) and dextromethorphan/quinidine (DM/Q) are known to be effective in the treatment of PBA symptoms.
18–20 The mood effects of DM/Q are not established, and our findings suggest the possibility that patients with parkinsonism could preferentially benefit from treatment with antidepressants for dual management of both PBA and mood symptoms with a single agent.
Interestingly, the present study further revealed that PBA symptom severity correlated with cognition in aP patients but not in PD or ALS patients. In our cohort, patients with aP tended to demonstrate more cognitive impairment (though not significant when corrected for age and education), but they were no more likely to fall into the PBA(+) category or experience worse severity of PBA symptoms. However, lower cognitive function was related to increased chance of PBA(+) group assignment in the aP patients, which was not observed in other clinical groups. This suggests that increased PBA symptoms may be related to declines in cognitive functioning that may occur earlier within atypical parkinsonism and may manifest later in the disease process with other neurodegenerative conditions. However, it is also possible that our study recruited insufficient numbers of individuals with PD and ALS with cognitive dysfunction and that disease-specific correlates change over time.
Although the pathophysiology of PBA remains poorly defined, dysfunction in multiple areas of the brain including the cortical mantel, subcortical white matter, basis pontis, and cerebellum have been implicated.
21 In view of these early neuroanatomical findings, the presence of PBA in neurological disorders with varied pathophysiology suggests there may be underlying differences in PBA characteristics or correlates. Despite obvious differences in pathophysiology compared with those with PD and aP, ALS subjects were not more likely to exhibit symptoms compatible with the diagnosis of PBA or experience worse PBA symptom severity. Additionally, although severity of depression and anxiety did not differ between disease groups, these were significant correlates of PBA symptoms among PD and aP patients but not ALS. Taken together, these findings support the notion of an alternate mechanism of PBA in ALS compared with parkinsonian disorders.
The present study included analyses using both raw and standardized scores on the MoCA. The standardized scores were calculated using population-based MoCA norms by Rossetti and colleagues.
16 As demonstrated in our results, these two scores provided similar results but with a few notable discrepancies (e.g., relationship between clinical group and cognition, relationship between PBA severity and cognition by clinical group). These discrepancies highlight an important though often overlooked consideration when using cognitive screeners: the potential impact of age and education on performance. Future studies should use normative data when analyzing MoCA scores in order to more accurately estimate an individual’s cognitive performance.
There are several limitations to this study. Although there was sufficient power to detect moderate effects, a larger sample would have increased the power to detect small effects, which may be clinically important and further delineate differences in PBA symptom pathophysiology in different populations. The validated CNS–LS for PBA was used in this study to indicate the presence of self-reported symptoms of affective lability (including pathologic laughter and crying) that could be compatible with the diagnosis of PBA, but a formal clinical interview was not used to verify a PBA diagnosis. Furthermore, self-reported questionnaires about mood symptoms were used rather than a formal clinical diagnostic interview to establish presence of a coexisting mood disorder. It is therefore possible that the full extent of symptom relationships or group differences was not appreciated. Another limitation for the study is the need to exclude patients with advanced disease, due to impaired language and communication skills and the lack of assessment of disease severity on PBA manifestations and correlates. It is possible that the proportion of PBA or the relevant clinical correlates may differ in more advanced stages of these conditions. PD is characterized with presynaptic degeneration of dopaminergic neurons in the substantia nigra, whereas the aP group of disorders have other postsynaptic dysfunction of similar basal ganglia circuits. Inclusion of multiple aPs into one group was made on this basis because of relatively small recruitment numbers, but it may have further prevented the opportunity to investigate disease-specific differences in PBA manifestations. Future research should include larger cohorts and a more thorough neuropsychological evaluation (including structured interviews to establish formal diagnoses) in order to fully understand the relative contribution of cognitive dysfunction and mood disorders in the manifestation of PBA symptoms across the full clinical spectrum of these conditions.
Conclusions
In summary, if cross-validated, the results of the current study suggest that PBA is related to greater levels of anxiety, depression, and lower mental health quality of life. Patients with PBA in the context of a parkinsonian disorder are more likely to exhibit such mood disturbances than those with ALS, a finding that may have implications for therapeutic decision-making. Furthermore, younger patients with high levels of anxiety are most likely to endorse PBA symptoms. Clinically, it is important for health care professionals to be aware of different correlates for symptoms of PBA in neurodegenerative disorders, in order to more appropriately diagnose and treat individual patients. The presence of mood disorders and cognitive dysfunction is of particular relevance and should be considered when features of PBA are suspected.