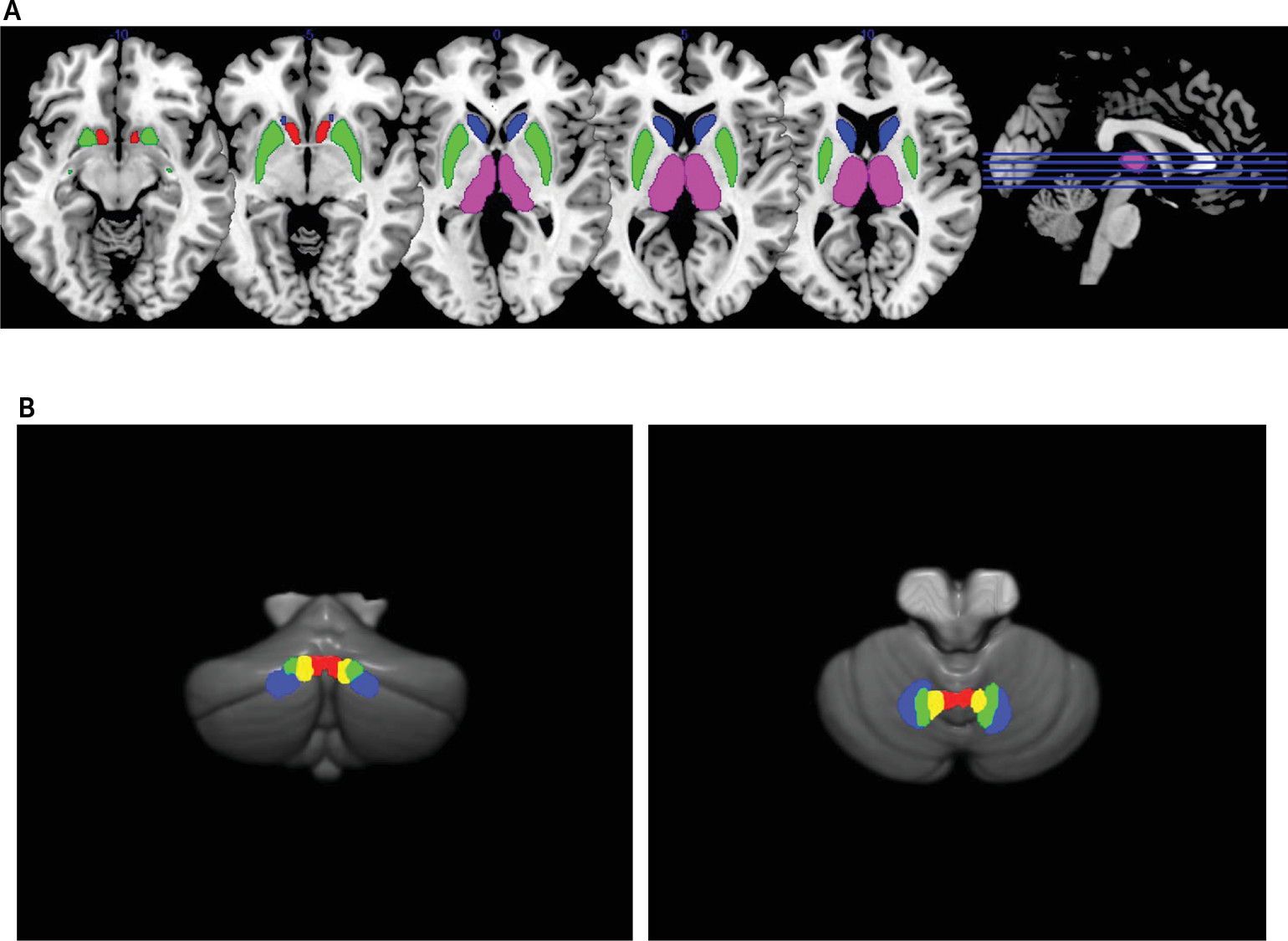
The striatum as part of the cortico-striato-thalamo-cortical circuitry may play a central role in schizophrenia pathophysiology, because the striatum is the primary target of all antipsychotics.
1 The dorsal striatum is associated with cognitive and motor functions in conjunction with dorsolateral prefrontal and sensorimotor areas, while the ventral striatum is related to emotional functions via its connections with limbic and orbitofrontal regions.
2 Excessive yet imbalanced dopaminergic activities in the striatum along the dorsal and ventral axis appear to be critical in understanding schizophrenia pathophysiology.
3 Nonetheless, the exact nature of striatal dysfunction in schizophrenia is still illusive, and it may be necessary but not sufficient to explain the neurobiological heterogeneity of schizophrenia.
Cerebellar efferents have their origin in the deep cerebellar nuclei, and they reach multiple subdivisions of the thalamus via the superior cerebellar peduncle (SCP) to modulate the cortico-striato-thalamo-cortical circuitry.
4 The dentate nucleus (DN), which is under the lateral hemisphere, is divided into dorsal and ventral portions (DNd and DNv), as DNd mainly projects to cortical motor areas via the motor thalamus whereas DNv is predominantly connected to prefrontal cognitive areas via the mediodorsal thalamus. Likewise, a more medially located nucleus under the paravermal zone, the interpositus nucleus (IN) has both anticipatory motor and cognitive functions.
5 Finally, the fastigial nucleus (FN) is located under the anterior vermis and has connections with ventral tegmental dopamine neurons targeting ventral striatal neurons to contribute to emotional functions.
6To date, evidence on the contribution of cerebellar efferents to the striatum, thalamus and cerebral cortex in schizophrenia is lacking. Although there have been some studies showing generally decreased resting-state functional connectivity (RSFC) between the cerebral cortex and cerebellar cortical areas in schizophrenia including antipsychotic-naïve cases.
7,8 It is important to investigate cerebellar efferents, because Purkinje cells, which provide the output of the cerebellar cortex to the deep cerebellar nuclei, have been reported to be impaired in terms of reduced cell size and density in schizophrenia.
9 Hence, it is likely that cerebellar input and output are dissociated. Resting-state fMRI provides an opportunity for such an investigation, as an in vivo examination of intrinsic brain organization.
10Here, we examined RSFC between cerebellar output nuclei and striatal regions using resting-state fMRI in patients with first-episode psychosis (FEP), to minimize the confounding effects of illness chronicity and long-term antipsychotic medication. In particular, we wished to determine the unique influences of each region to the whole brain that are not mediated by other regions, using a seed-to-voxel semipartial correlation analysis. We also examined the integrity of cerebellar white-matter output tracts (the SCP) using diffusion tensor imaging (DTI) and cerebellar and striatal gray-matter volumes for possible structural-functional constraints, as we sought a convergence of evidence. The functional significance of these measures would be examined using a cerebellar-sensitive cognitive measure of verbal fluency
11 and clinical ratings. We hypothesized a relative hyperconnectivity between cerebellar output nuclei and striatal regions in FEP, based on evidence for excessive cerebellar activity
12 and also based on hyperactivity in the striatal dopaminergic system in schizophrenia.
1Results
ROI Connectivity Analyses
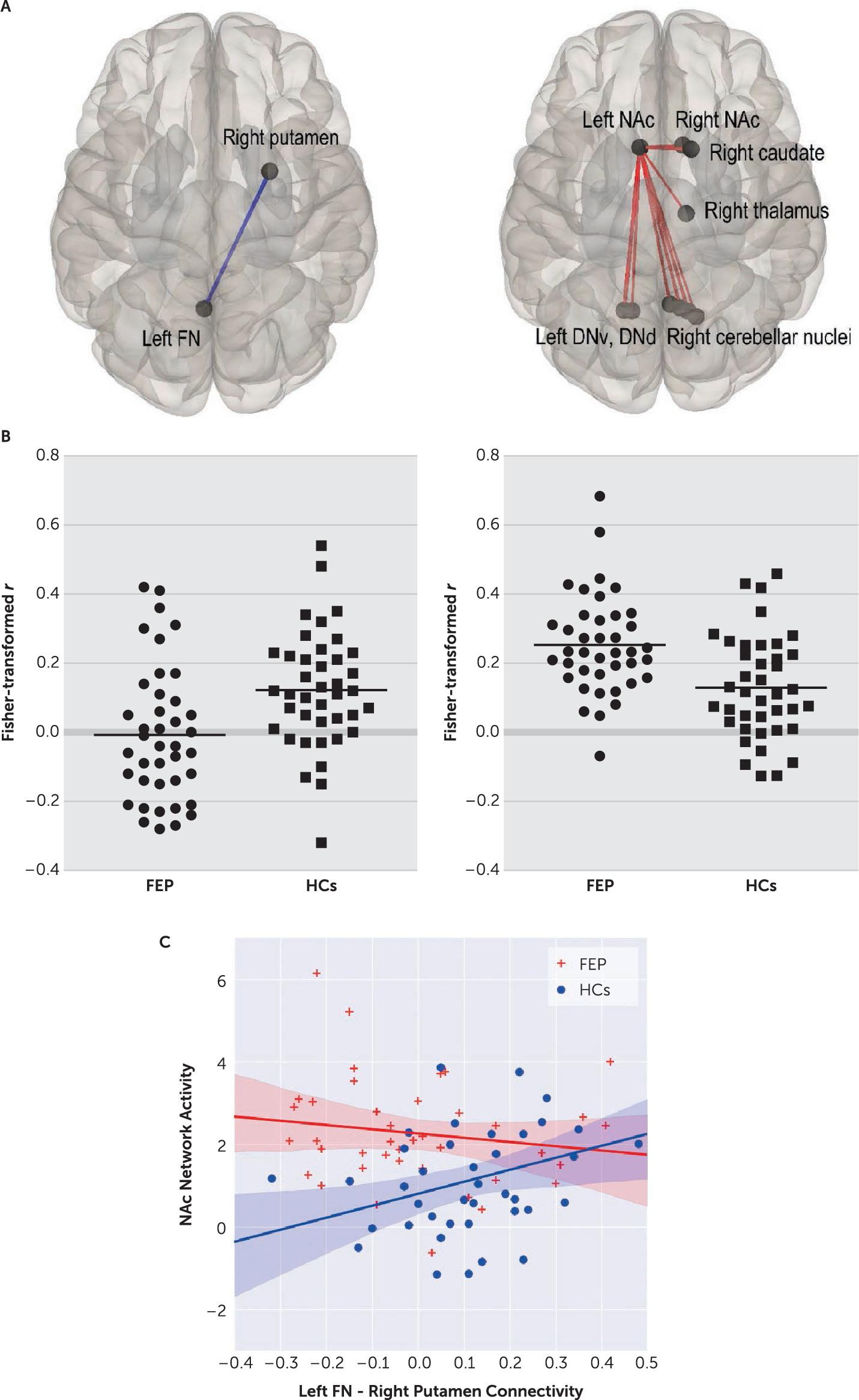
Patients exhibited significantly decreased RSFC between left FN and right putamen ROIs, compared with HCs (t=–3.19, df=78, p=0.026, corrected) (
Table 2,
Figure 2). This RSFC strength was positively correlated with letter fluency performance in patients (r=0.36, p=0.021; but not in HCs: r=−0.109, p=0.539), and global assessment of functioning (GAF) scores in patients (both current and past year: r=0.38, p=0.015, and r=0.40, p=0.010).
At network level, we found a significantly increased intensity of left nucleus accumbens (NAc) network in patients, compared with HCs (p=0.035, FWE-corrected). This network comprised of individual connections of all cerebellar output nuclei, except for the left FN and IN, to the left NAc. Left NAc connections with the right NAc, caudate and thalamus were also included (
Figure 2 right column). This network activity was negatively correlated with letter fluency performance that fell short of statistical significance among patients (r=−0.269, p=0.094).
HCs showed a positive correlation between the left FN and right putamen connectivity and the left NAc network activity (r=0.358, p=0.023), which was absent in patients (r=−0.152, p=0.349) (
Figure 2C). Within patients, these connectivity results were not significantly associated with any of three syndrome scores. Individual symptoms associated with these results were ‘difficulty in abstract thinking’ (with left FN and right putamen connectivity: r=−0.388, p=0.013) and ‘excitement’ (with the NAc network activity: r=0.341, p=0.031).
Unique Functional Connectivity Analyses
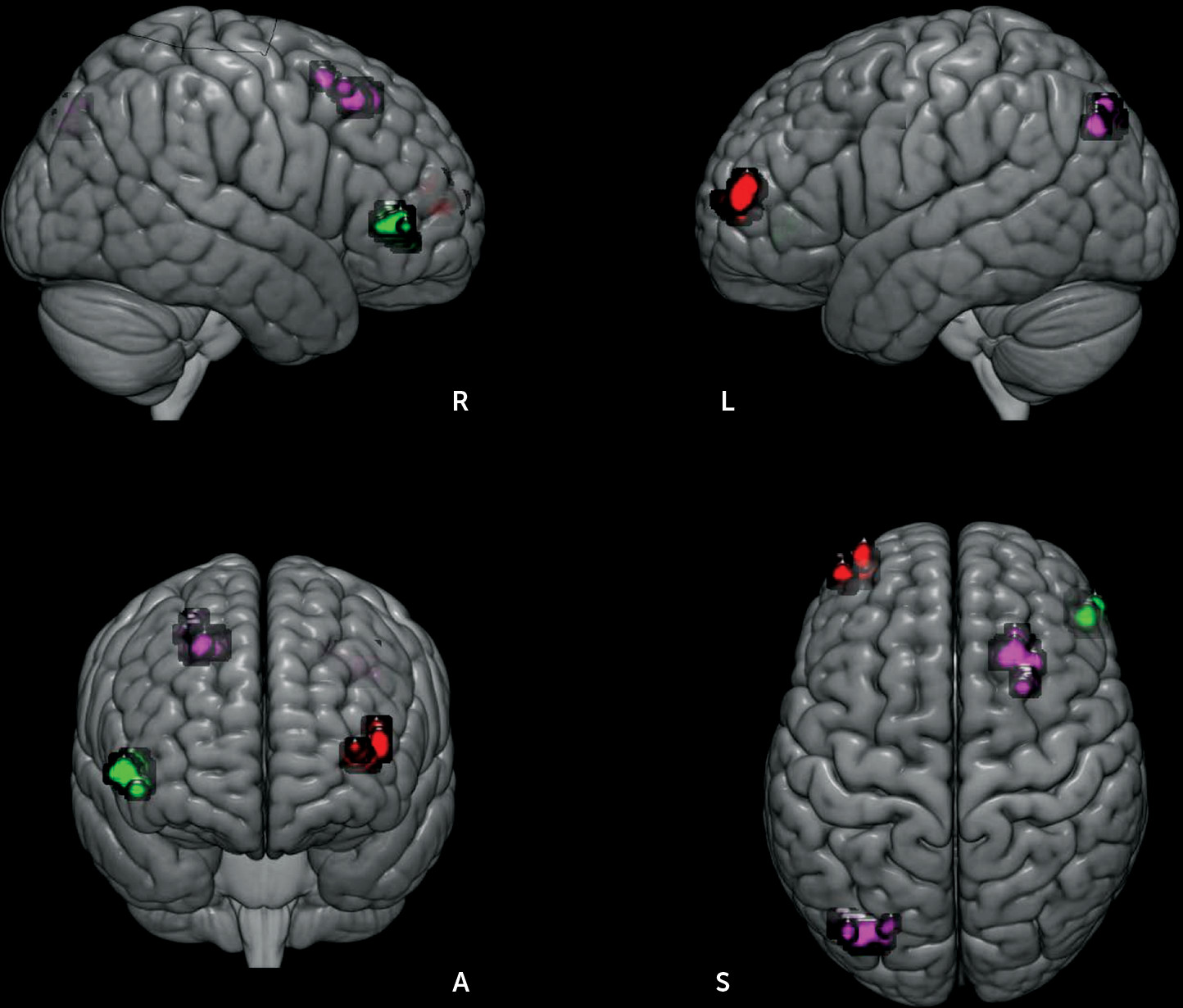
Each ROI’s unique influence to the whole brain was examined by controlling for the contributions from other ROIs. As shown in
Figure 3, patients exhibited decreased RSFC between the left IN and the right-sided homolog of Brodmann’s area (BA 45). By contrast, increased RSFC of the right IN with left inferior parietal cortex (BA 39, angular gyrus) and right premotor cortex (BA 6), and increased right DNv connectivity with left lateral frontopolar area (FP1, BA 10) were observed in patients. The unique connectivity increase was associated with disorganization symptoms (right IN connectivity with the left inferior parietal cortex: r=−0.462, p=0.003; right DNv with left lateral frontopolar area: r=0.338, p=0.033).
DTI Findings
We found significantly increased axial diffusivity and an increase in RD values that fell short of statistical significance in the left SCP in patients (t=3.03, df=78, p=0.004; t=1.731, df=78, p=0.088), where both measures were highly positively correlated with each other (r=0.422, p=0.007). Hence, left SCP mean diffusivity values were significantly increased in patients (t=2.26, df=78, p=0.026). FA values for the left SCP were not different between groups (t=0.707, df=78, p=0.482). In addition, we found a level of increased right SCP FA values that nearly reached statistical significance among patients (t=1.745, df=78, p=0.085), which were positively correlated with current GAF scores (r=0.332, p=0.036). The means and standard deviations of DTI variables for three pairs of cerebellar peduncles were documented in Table S2 in the online supplement.
Volumetric Findings
Means and standard deviations for all volumetric variables were presented in Table S3 in the online supplement. Head-size-corrected (regional volume divided by intracranial volume) bilateral putamen (left: t=2.41, df=78, p=0.019; right: t=2.01, df=78, p=0.048), left NAc (t=1.78, df=78, p=0.079), left anterior cerebellar volumes (t=1.90, df=78, p=0.061) were significantly or marginally significantly decreased in patients, compared with HCs. Psychomotor poverty scores were positively correlated left putamen volume (r=0.349, p=0.027).
Correlations Among Neuroimaging Variables
RSFC abnormalities in patients were not correlated with any DTI or volumetric measures in patients. Left putamen volume was positively correlated with left anterior cerebellar volume in patients (r=0.542, p=0.0003), but negatively correlated with right SCP FA values (r=−0.371, p=0.018) and right IN unique connectivity with right premotor area (r=−0.321, p=0.044) in patients. These correlations are consistent with factor analytic results (for further details, see Table S4 in the online supplement). These correlations were not found in HCs.
In HCs, the left FN and right putamen connectivity was positively correlated with bilateral putamen (left: r=0.381, p=0.015; right: r=0.409, p=0.009) and left NAc (r=0.332, p=0.036) volumes. Left anterior cerebellar volume was positively correlated with right SCP FA values (r=0.368, p=0.019). Yet, these associations were not found in patients.
Discussion
Our results provided evidence for disordered RSFC between cerebellar output nuclei and striatal regions at the early stage of schizophrenia. Furthermore, we demonstrated that cerebellar output nuclei exert dysfunctional influences to fronto-parietal areas in patients, which is independent of striatal dysfunction. Finally, we highlighted that a normal structural-functional relationship is impaired in patients, by examining RSFC associations with DTI and volumetric measures.
Patients exhibited decreased RSFC between left FN and right putamen ROIs. The FN is critically involved in autonomic and oculomotor functions, that are well-known to be impaired in schizophrenia.
23 In experimental animals, electrical stimulation of the FN increased blood flow in the dorsal striatum, sensorimotor and fronto-parietal areas, with a long-lasting neuroprotection effect against excitotoxic lesions of the striatum induced by a psychostimulant.
24 In turn, the right putamen has been part of fronto-parietal cognitive network that is significantly underactivated in schizophrenia patients during executive function tasks including verbal fluency.
25 This decrease of left FN and right putamen connectivity was associated with poor letter fluency performance, and also with lower GAF scores (both current and past year’s) in patients. This RSFC deficit may therefore represent a biomarker associated with executive dysfunction and poor outcome in patients.
Patients showed a widespread increase of the left NAc network. This hyperconnectivity also appeared to be dysfunctional, because letter fluency performance decreased (at a level that fell short of statistical significance), as the network activity increased. The conflicting hypo- and hyperconnectivity results are also commonly reported in both first-episode and chronic schizophrenia patients groups.
26 Abnormally increased RSFC might reflect a compensatory, plasticity response to a primary pathophysiological process producing a disruption of topography of RSFC.
27 Rather, we propose that our finding of both hypo- and hyperconnectivity results from different pathological sources. Our correlation and factor analysis results support our proposal, as they were unrelated to each other in patients (for further details, see Table S4 in the
online supplement). These results have important implications for repetitive transcranial magnetic stimulation studies targeting the cerebellar vermis (and underlying the FN) using a facilitation protocol in schizophrenia.
28 The mechanism of action in reducing symptom severity appears to be ameliorating functional connectivity decrease (e.g., left FN and right putamen connectivity as in our study) associated with cognitive and functional impairments. Then, a high level of excitement (e.g., agitation) would be a contraindication to such a treatment, as shown in our symptom correlation results.
We used a seed-to-voxel semipartial correlation analysis to investigate between-group differences in unique RSFC between our ROIs and the whole brain voxels. This semipartial correlation analysis, similar to multiple regression analysis, assesses a seed’s ‘unique’ part of the total variance at each voxel, relative to the residual, in the presence of signals from other seeds.
29 Our results suggest that most if not all striatal-cortical RSFC abnormalities in patients are accounted by abnormal cerebellar influences, because no striatal seeds produced any significant between-group differences. It is particularly noteworthy that patients displayed significantly decreased unique RSFC between the left IN and right Broca’s area. It is well established that the IN, together with Broca’s area (BA 44/45), plays an important role in the timing of delay eye blink conditioning and in various timing tasks.
5,30 Delay eye blink conditioning and timing functions are impaired in both schizophrenia patients and their first-degree relatives.
31–33 The interplay between cerebellar and prefrontal areas appears to be critical for timing dysfunctions in schizophrenia.
We sought converging evidence from RSFC, DTI and volumetric investigations, which would be a distinct strength of our study. We did so, because there would be a possible compensatory increase of RSFC in case of gray-matter loss and/or impaired white-matter integrity.
34 Two correlation results are of particular mention in this respect. Neither left anterior cerebellar nor right putamen volume was associated with the decrease of left FN and right putamen RSFC in patients. By contrast, this left FN and right putamen RSFC was positively correlated with bilateral putamen and left NAc volumes in HCs. The other important correlation is relating to the left NAc network activity which was increased in patients. Because left NAc volume was decreased at a level that fell short of statistical significance among patients, one would expect a significant negative correlation between left NAc volume and the strength of NAc network activity. However, it was not the case in our results. Taken together, these results indicated that normal structural-functional relationships are lost in patients. Consistent with this, studies investigating a structure-function relationship using DTI and resting-state fMRI have not found correspondence between the two measures in chronic schizophrenia patients.
35 We suggest that multiple brain abnormalities from different origins do not converge even at the chronic stage of this illness.
A reduction in white-matter integrity in the left superior cerebellar peduncle (SCP) was found in patients, in terms of increased axial diffusivity, radial diffusivity (RD at level that fell short of statistical significance) and mean diffusivity. Increased axial diffusivity has been seen in an end state of axonal damage (i.e., when axon fragments are cleared), while increased RD has been related to demyelination.
36 Although the exact meaning of this finding would need to be investigated in future studies, a number of previous studies have reported abnormalities that are specific to the SCP in chronic schizophrenia patients.
37,38 The majority of these reported predominant left SCP abnormalities. Furthermore, adolescents at high risk for psychosis progressively exhibited reduced FA values in the SCP (left more than right) over 12 months.
39 However, the middle cerebellar peduncle (MCP) which carries input fibers from the contralateral cerebral cortex to the cerebellar cortex via the pons was not significantly lowered in adolescents with first-admission or chronic schizophrenia patients.
40,41 We also documented that the MCP was not disordered in our patients (for further details see Table S2 in the
online supplement).
There are further issues to consider in interpreting our results. First, we did not perform statistical correction procedures for possible type I errors for our correlation analyses and between-group analyses for DTI and volumetric variables. When significant (or near-significant) between-group differences were found, we sought to find their cognitive, symptomatic and functioning correlates (84 comparisons). Thus, our correlational and DTI and volumetric between-group results must be interpreted with caution. Nonetheless, they may provide a basis for interpreting our neuroimaging data and generating new hypotheses. Second, our ROIs did not include cerebellar cortical areas for investigating cortico-ponto-cerebellar cortical connectivity (mediated by the MCP), because the most inferior edge of the cerebellar cortex was not covered in our fMRI scan for approximately 20% of participants (but the cerebellar nuclei were covered in all participants). We did not want to introduce a selection bias based on head-size. Moreover, our focus was on the influence of cerebellar output nuclei to the striatum and cerebral cortex, as available cerebellar DTI studies to date reported predominant abnormalities in the SCP (consistent with our DTI results, see Table S2 in the online supplement). Thirdly, special care was taken to minimize a possible signal contamination from neighboring ROIs. We could not completely rule out this possibility for cerebellar nuclei due to their spatial proximity. Nonetheless, our specific RSFC patterns did indicate that signals from different cerebellar nuclei were independent from each other, displaying different connectivity profiles. Finally, future studies for cerebellar functional connectivity may include timing tasks that are sensitive to cerebellar impairment (Lee et al., 2009, 2007).
In conclusion, our study provides evidence that RSFC between cerebellar output nuclei and striatal regions is disordered in patients at the early stage of schizophrenia. We found that dysfunctional cerebellar influence to the whole brain is above and beyond that of the striatum in patients. We further present comprehensive descriptions for abnormalities in cerebellar influence to striatal and cerebral cortical areas by examining RSFC, DTI, and volumetric data in the same group of first-episode psychosis patients. As such, our study provides a basis from which future investigations on cerebellar-striatal interaction abnormalities in schizophrenia are performed.