A history of moderate to severe traumatic brain injury (TBI) has been linked to the development of neurodegenerative conditions, including Alzheimer’s disease (AD) (
1) and all-cause dementia (
1–
3). Some studies suggest that TBI may be associated with accumulation of amyloid-beta plaques (
4,
5) and neurofibrillary tangles (
5) and that TBI with loss of consciousness (LOC) is associated with earlier age at Alzheimer’s onset by 2.5–3.5 years (
6,
7). Nevertheless, the mechanism by which TBI increases the risk and earlier onset of AD or dementia is unknown and may involve the initiation or acceleration of a neurodegenerative process or a lowering of cognitive or neuronal reserve (for a detailed review, see reference
8). However, not all studies have found a significant association between TBI and AD or dementia (
9,
10), and more investigations are needed to determine mechanisms and identify specific groups that may be more at risk of AD after TBI.
Previous studies on the long-term effects of TBI and pathology or dementia resulting from AD are largely based on Caucasian samples, despite the accelerating growth of the racial-ethnic minority population in the United States (
11). In addition, Hispanic and African-American populations have a higher prevalence of AD, compared with non-Hispanic Caucasians, with rates 1.5 and 2 times higher (
12,
13). Hispanic ethnicity has been associated with a higher risk of earlier AD onset (
14), and African Americans have been identified as having a higher risk of developing AD, both compared with non-Hispanic Caucasians (
11). Recently, a small sample of Mexican Americans (N=22) showed greater severity of cognitive impairment and comorbid depression at initial visit to a memory disorders clinic, compared with a non-Mexican cohort (
15). Although factors such as education, gender, hypertension, hypercholesterolemia, and diabetes have been associated with this increased risk, TBI as a risk factor for dementia in racial-ethnic minority populations in general has yet to be explored (
16). Because a history of TBI has been linked to an increased risk of earlier onset of AD and related conditions in primarily Caucasian samples (
6–
8,
17), examining whether a similar association is found in individuals of Hispanic ethnicity may have implications for improving our understanding of dementia onset in these traditionally understudied populations.
This study aimed to assess estimated age at AD onset between individuals with and without a history of TBI among three racial-ethnic cohorts (African American, non-Hispanic Caucasian, and Hispanic). The study also evaluated the influence of other potential dementia risk factors including education, gender, stroke history, depression history, number of apolipoprotein E (APOE) ε4 alleles, diabetes, hypertension, hypercholesterolemia, and family history of AD.
Methods
Participants
Data were obtained from the National Alzheimer’s Coordinating Center (NACC) uniform data set (UDS). The UDS has been aggregating sociodemographic, clinical, and medical information data on adults with subjective memory concerns, mild cognitive impairment, and various neurodegenerative dementias since 2005 from National Institute on Aging–funded Alzheimer’s Disease Research Centers (ADRCs) nationwide. Written informed consent was obtained from participants at each ADRC and approved by each center’s institutional review board. Research using the NACC database was approved by the institutional review board at the University of Washington. Data for the study reported here were derived from 32 ADRCs across the United States from September 2005 to March 2015. NACC UDS versions 1 and 2 were exclusively used to maintain consistent TBI criteria across samples. Inclusion criteria for the study reported here were as follows: age ≥50 years, clinical diagnosis of dementia due to AD at an initial or follow-up ADRC visit, available data on self-reported race and ethnicity and TBI history, and clinician-estimated age at cognitive symptom onset. A multidisciplinary team of clinicians reached a consensus diagnosis of AD by using standardized criteria. Diagnosis of AD was made according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (
18) criteria. Race and ethnicity data were gathered through subject and informant report, and groups were categorized as non-Hispanic Caucasian, African American, and Hispanic.
Measures
A history of TBI was defined by combining several variables within the NACC data set. In NACC UDS versions 1 and 2, study subjects and informants were asked whether the subject had ever sustained a TBI with LOC lasting <5 minutes or ≥5 minutes or whether the injury resulted in chronic deficit or dysfunction. Each of these responses was coded as recent-active (i.e., occurred within the past year or still required active management), remote-inactive (i.e., occurred >1 year ago but was resolved or with no current treatment under way), or absent. In UDS versions 1 and 2, date of injury was not available aside from more than or less than 1 year prior to an ADRC visit. Because <5 or ≥5 minutes of LOC is an arbitrary cutoff that does not follow any clinically established guidelines for severity, we combined these variables into TBI with any duration of LOC. To minimize the possibility that recent injuries affected the subject’s diagnosis of dementia, we included only those who sustained a TBI with LOC more than 1 year prior to their diagnosis of dementia and had no chronic cognitive deficits from injury (TBI+). Those who reported an absence of a history of TBI with LOC during their visit were included as the comparison group (TBI–).
Available clinical data to guide diagnosis of dementia included information gathered from the subject or informant, medical records (i.e., neuropsychological and neurological examination findings and detailed medical history), or observation. Clinician-estimated age at onset of dementia due to AD was guided by clinical judgment informed by temporal correlation of the totality of the aforementioned data. The age at onset was estimated when the subject was first diagnosed as having AD (regardless of dementia status). Therefore, all study participants were diagnosed as having dementia due to AD either at the first visit or at subsequent visits.
Several risk factors for AD have been shown to have varying prevalence rates among different racial and ethnic groups. Therefore, between the TBI+ and TBI– groups, we evaluated whether gender; education; number of APOE ε4 alleles; family history of dementia; or history of depression, hypertension, hypercholesterolemia, diabetes, or stroke could have a potential influence on the age at onset. For this study, history of hypertension, hypercholesterolemia, diabetes, and stroke were coded in NACC as absent, recent-active, or remote-inactive and were dichotomized into present (recent or remote) or absent conditions. Depression and family history of dementia (i.e., first-degree relative) was coded as absent, present, or unknown in the NACC database. Depression was defined in NACC as having been diagnosed with a mood disorder (major depression, dysthymia, or bipolar disorder) with DSM-IV-TR or DSM-5 criteria, evaluated by a clinician for depressed mood, or prescribed an antidepressant medication.
Statistical Analysis
Analyses were conducted separately for the three cohorts (non-Hispanic Caucasians, African Americans, and Hispanics). Independent-samples t tests and chi-square analyses, where appropriate, evaluated whether TBI+ and TBI– groups differed in years of education; sex; number of APOE ε4 alleles; family history of dementia; or history of depression, stroke, hypertension, hypercholesterolemia, and diabetes. Variables having significant differences were entered as covariates in the primary analyses. Analyses of covariance (ANCOVAs) were used to assess whether dementia onset differed between TBI+ and TBI– groups, and we assessed for interactions between TBI history and any covariate within the ANCOVA models. If a significant interaction was found, the model was run as a 2×2 ANCOVA. Levene’s test was used to assess for equality of variances between groups. If significant, nonparametric Welch analyses of variance (ANOVAs) were used to assess whether significant differences remained after accounting for unequal variances. Missing data were excluded case-wise for each individual analysis. Level of significance was set at p<0.05 for all analyses, and analyses were carried out using IBM Statistics V24.
Results
Of the 9,683 participants who met initial inclusion criteria, 84% (N=8,134) were diagnosed as having dementia secondary to AD at the first ADRC visit, and the others (16%, N=1,549) were eventually diagnosed as having AD. Data on informant sources were available for 9,594 study subjects, of which 92% (N=8,804) of informant reports came from a close family member (spouses or partners, 58%, N=5,606; children, 31%, N=2,961; and siblings, 3%, N=237). Missing data for the entire sample were as follows: stroke, N=36, <1%; depression, N=110, 1%; APOE ε4 alleles, N=2,319, 24%; diabetes, N=22, <1%; hypertension, N=23, <1%; hypercholesterolemia, N=100, 1%; and years of education, N=80, <1%. The final sample of the non-Hispanic Caucasian cohort included 669 study subjects with a history of TBI with LOC (TBI+) (8.8%) and 6,908 without a history of TBI with LOC (TBI–) (91.2%). In the African-American cohort, 46 study subjects (5.9%) had a history of TBI with LOC and 730 (94.1%) did not. In the Hispanic cohort, 60 participants (6.9%) reported TBI with LOC and 810 (93.1%) did not.
In the non-Hispanic Caucasian cohort, significant differences were found between the TBI+ and TBI– groups in years of education (p=0.021), sex (p<0.001), and remote depression (p=0.011) (
Table 1). In the African-American cohort, significant differences were found between the TBI+ and TBI– groups in sex (p=0.008) and number of
APOE ε4 alleles (p=0.029). In the Hispanic cohort, significant differences were found between the TBI+ and TBI– groups in education (p=0.011) and sex (p<0.001).
A series of ANCOVAs were carried out to assess whether estimated AD onset differed between TBI+ and TBI– groups in each cohort (
Table 2). In the non-Hispanic Caucasian cohort, onset of AD occurred 2.30 years earlier for the TBI+ group, compared with the TBI– group (F=30.49, df=1, 7,572, p<0.001). However, Levene’s test revealed a violation in the assumption of homogeneous variances; thus a nonparametric Welch ANOVA was conducted, and a significant difference between TBI+ and TBI− groups remained (F=29.32, df=1, 7,697, p<0.001). Similarly, in the African-American cohort, onset of AD occurred 3.37 years earlier for the TBI+ group, compared with the TBI– group (F
=5.17, df
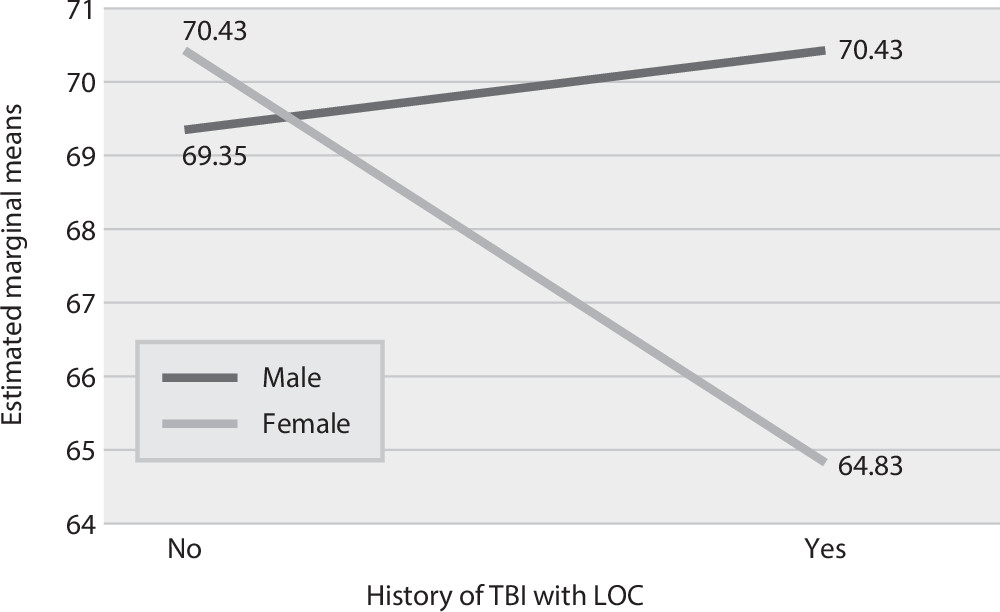
=1, 772, p=0.023). In the Hispanic cohort, a significant interaction was observed between sex and TBI history (F=6.96, df=1, 865, p=0.008), indicating that for females in the TBI+ group, the onset of AD occurred 5.6 years earlier, compared with females in the TBI– group; little difference was observed for males with and without a TBI history (
Figure 1). No other significant interactions were observed between TBI group and covariates in any racial-ethnic cohort.
Discussion
To our knowledge, this study is the first to examine the risk of earlier AD onset after TBI among different racial and ethnic groups. We found that a TBI history with LOC was associated with an onset of symptoms of 2–3 years earlier for both the non-Hispanic Caucasian and African-American groups. Although this association is similar to previous findings in samples with mild cognitive impairment (
17) and autopsy-confirmed AD (
6), those studies were predominantly of non-Hispanic Caucasian samples (≥80%). Thus the findings reported here suggest that the risk of earlier onset of AD is comparable for non-Hispanic Caucasians and African Americans with a history of TBI with LOC. This finding adds to a growing literature indicating that TBI might be linked with accelerated aging or earlier onset of dementia secondary to AD. However, in the Hispanic group, a history of TBI with LOC was associated with an AD onset nearly 6 years earlier among females but not among males. It is possible that Hispanic females possess some genetic factor(s) leading to a vulnerability to TBI effects, and protective factors in Hispanic males may offset any effects from a history of TBI, although several other factors may be contributing to this relationship.
It is possible that Hispanic females in this sample may be more prone to earlier AD onset after TBI because of a combination of factors related to the interaction of cultural factors and treatment seeking from medical care providers, unique TBI risk factors, and socioeconomic standing. In a small subset of patients who presented to a memory disorders clinic, Mexican Americans presented later in the course of dementia and with greater comorbid depressive symptoms, compared with Caucasians, but the clinical rating of dementia severity was similar to that of Caucasians (
15). These findings could be a result of Hispanics presenting to clinical attention with greater cognitive impairment than Caucasians but with similar functional ratings by clinicians, which may be related to a mediating factor of family support. A 2015 review of the influence of social-familial-financial factors on the well-being of Hispanics may shed light on our findings (
19).
Other potential contributors to our findings in the Hispanic cohort may be more severe or frequent head injuries, which could not be examined further in this study, and factors that impede Hispanic individuals from seeking health care after injuries. A national U.S. survey (
20) found that 15.6% of Latino women endorsed a history of intimate-partner violence, which is supported by findings that long-term Hispanic and mixed-race couples have the highest rates of intimate-partner violence (
21). This is within the range of lifetime prevalence of intimate-partner violence among U.S. females, which is estimated at 25%. However, nationwide rates of intimate-partner violence among females in Mexico were reported to be significantly higher at 45% (
22). Female victims of intimate-partner violence are at higher risk of repetitive TBI with LOC (
23) and the possibility of hypoxia secondary to strangulation (
24). Thus Hispanic women may be more likely to sustain more frequent or more severe TBI, which may put them at higher risk of earlier AD onset postinjury. In addition, the intersection of sex and cultural factors (i.e.,
Marianismo, in which women are taught to be modest, virtuous, and faithful and subordinate to their husbands) could have affected self-reported history of TBI, partially accounting for the difference in the Hispanic cohort.
Finally, income disparities may make Hispanic females less likely to receive health care or to have the health literacy required to enact lifestyle changes (i.e., physical exercise) to reduce their risk of dementia. This study added education as a covariate, but no data on income and occupational status were available in the data set. However, because of the lack of available data on age, chronicity and severity of injury, and socioeconomic and lifestyle factors, it is unknown whether these factors might have contributed to our findings. In addition, these factors do not account for the lack of significant findings among Hispanic males versus non-Hispanic males. Clearly, future study of dementia risk factors among Hispanic cohorts is needed.
Our study has several limitations. First, the sample sizes of the Hispanic and African-American groups with a history of TBI were small, which may affect generalizability. As previously noted, the temporal relationship of reported TBI and cognitive impairment is confounded by the possibility that a head injury may have occurred following the onset of cognitive impairment (
6), and, unfortunately, date of injury is not recorded in UDS versions 1 and 2. However, to minimize the possibility that the TBI occurred after onset of cognitive impairment and to reduce the possibility that TBI confounded the diagnosis of dementia, we included only study subjects with remote injuries (>1 year prior to an ADRC visit) without reported chronic deficits. Other study limitations include self-report of TBI and lack of TBI characteristics (e.g., severity and posttraumatic amnesia), although it is likely that injuries were milder in this study given the lack of reported chronic deficits. In addition, significant heterogeneity may exist in the Hispanic cohort, such as level of acculturation, bilingualism, generation in the United States, and age at immigration, which could have affected our findings.
Despite these limitations, this is the first study, to our knowledge, to approach TBI history as a risk factor for earlier AD onset in racially and ethnically diverse cohorts, resulting in intriguing preliminary findings related to sex differences in a Hispanic cohort. We are cognizant of the potential for our hypotheses regarding these observed differences to be interpreted as an overextension of the data. After careful consideration, we decided that the benefit of highlighting these concerns is greater than the cost of perpetuating the invisibility of crucial factors in an increasingly racially and culturally diverse world. An obvious need exists for national databases to include specific information regarding TBI (e.g., date of injury, injury characteristics, and severity) and important cultural variables (e.g., acculturation level and degree of bilingualism), along with in-depth collection of psychosocial information and lifestyle factors to promote more diversity-focused research in this area (
25).