Right-hemisphere stroke patients with anosognosia for hemiplegia (AHP) are unaware of their motor deficit and verbally deny that the left side of their body is weak. At the patient’s bedside, AHP may manifest in several ways. When asked to raise the paralyzed arm, some patients may instead raise their normal arm (allochiria) or do nothing at all, while other patients put great effort into moving but to no avail (
1). When asked whether the paralyzed arm has moved, the patient may say “yes,” claiming a movement that did not occur (
2). Verbal claims of movement have been noted since early reports of AHP, and most investigators categorized these responses as confabulatory. However, recent research in the area of motor perception has presented an alternative hypothesis: that patients with AHP experience an illusory sense of movement and that this kinesthetic experience may form the basis of the disorder (
3,
4). In the present study, we sought to test this hypothesis by asking patients with AHP about their kinesthetic experience during movement attempts.
The brain programs muscle commands and uses a copy of these commands, the efference copy, to inform other areas of the brain about the predicted sensory consequences of an intended movement (
3). The predicted state, defined by this efference copy, is continually compared with the actual state of the body, which is defined by the actual sensory feedback of the movement. A general concept in sensory physiology is that the contents of conscious perception are generated by the predicted state (
5). People may only be aware of the actual sensory consequences of their movements when there is a mismatch with what they expect (
6).
Within this framework, two proposals have been put forward to explain AHP. The feedforward hypothesis suggests that patients with AHP have defective intention to move, and thus no mismatch occurs between the predicted and feedback states (
7). Hence the person does not interpret the lack of movement as aberrant and denies paralysis. An alternative hypothesis suggests that patients with AHP have intact intention but have damage to the system that allows them to be aware of mismatch states—a comparator system that compares intended movement and the actual movement that occurred. If these comparator systems are damaged, then the patient’s awareness is dominated by intention—the efference copy provides the false sense of kinesthesia (
3).
Because first-person phenomenological accounts and neurocognitive models mutually inform each other, a more refined understanding of the basic phenomenology of AHP is likely to help improve models of its pathogenesis (
8). This kind of approach has been used for other neurological disorders such as vertigo. For example, a patient with vertigo as a result of an acute cerebellar stroke experiences the illusory perception of self-motion (the neurological symptom), and the examiner observes the direction of the patient’s nystagmus (the neurological sign) (
9). This bedside phenomenology not only aides the clinician in diagnosis but also provides important constraints on theoretical models of vestibular dysfunction; the models must account for the signs and symptoms of disease.
We applied this framework to AHP. The bedrock studies on the incidence, clinical characteristics, and diagnostic interview of AHP were conducted decades ago, predating the modern conceptions of the disorder (
10–
12). These studies also predated the modern era of hyperacute (<6 hours) stroke treatment in which patients are examined shortly after infarct, when AHP is most robust. Although high-quality interview measures were used in these previous studies, the resultant scales (e.g., the Bisiach Scale) collapse a multidimensional disorder into a flattened severity score, losing important phenomenological details (
10,
13).
The kinesthetic senses are the senses of position and movement of the body, senses that people are aware of only on introspection (
5). Whether patients with AHP experience kinesthetic illusions can only be addressed by a patient’s verbal account of his or her bodily experiences. To our knowledge, only two studies have addressed this specifically: one conducted by Lu et al. (
14), who examined this in five AHP patients undergoing the Wada test, and another conducted by Feinberg et al. (
15), who examined five poststroke AHP patients. Both groups of investigators found that claims of movement occurred in some but not all patients with AHP. The prevalence of movement claims in patients with AHP is unknown.
In addition, as pointed out by Moro et al. (
16), AHP has been investigated mainly by interviewing patients rather than by direct behavioral observations of their action or attempt to act. Observable neurological signs related to AHP have gone underexplored, yet they may provide relevant information about the nature of a patient’s unawareness (
17). Therefore, in the present study we not only concentrated on verbal accounts (patient symptoms), we also focused on nonverbal components (neurological signs) of disease manifestation as they relate to spatial cognition (neglect), intention, and implicit knowledge of impairment.
The nature of spatial neglect’s contribution to AHP has been a source of controversy. We surmised that the so-called productive manifestations of neglect may provide some window into neglect’s contribution to AHP (
18). These manifestations include allochiria, which is when a left-sided stimulus is reported on the right side of the body or space, or left-sided motor intention is translocated to the right (also referred to as allokinesia) (
2,
19). It is unknown how often patients display these aspects of strong spatial bias while responding to questions about their left hemiplegia or what effect this bias may have on a patient’s denial of weakness.
As previously mentioned, the nature of intention as it relates to AHP has also been the source of debate. The will to move and the subsequent proprioceptive sensations are intimately linked (
5). Yet the prevalence of intact or defective intention has not been adequately studied in AHP patients, nor has the prevalence of implicit knowledge.
It is clear that some patients who deny paralysis still retain access to some type of implicit knowledge about their motor impairment—that is, “knowledge that is expressed in task performance unintentionally and with little or no phenomenal awareness” (
20). Implicit knowledge is illustrated by the inconsistency between the patient’s verbal answers and behaviors, as when the patient asserts that he or she is able to walk yet at the same time refuses to leave the wheelchair (
13). Time-consuming test batteries have been developed to diagnose the presence of implicit knowledge in patients with AHP, but the systematic exploration of directly observable evidence of implicit knowledge at the bedside has largely not taken place (
17,
21).
Given this, we sought to reappraise the basic verbal and nonverbal clinical phenomenology of AHP. Thus, we prospectively examined the characteristics, natural history, and anatomical basis of movement claims and observable behavior during movement attempts (allochiria, intention, and implicit knowledge) among AHP patients with acute or hyperacute right-hemisphere stroke.
Methods
Participants
We prospectively screened consecutive stroke patients admitted to Montefiore Medical Center (Bronx, N.Y.). Inclusion criteria were left hemiplegia or severe hemiparesis, as defined by a NIH Stroke Scale arm score of 4 (no movement) or 3 (no effort against gravity) (
22); anosognosia, as defined by a Bisiach Scale score of 2 or 3 (
23); and acute right-hemisphere ischemic stroke or intracerebral hemorrhage on CT or MRI. A total of 29 out of 89 patients were enrolled for participation in the study. This study was approved by the institutional review board at Montefiore Medical Center, and informed consent was obtained from all participants.
Procedure
Patients underwent a battery of tests, including standard neurological examination, assessment with the NIH Stroke Scale, visual-spatial neglect testing (letter version of Albert’s cancellation task) (
24), asomatognosia testing (misidentification of the left arm), and AHP interview. After the first examination, patients were re-examined as often as possible until the day of discharge or 7 days had passed, whichever came first.
AHP was examined by using a modified version of Berti’s method (
25). This version includes a set of general questions (e.g., “Why are you in the hospital?”); specific questions about motor ability (e.g., “Is your left arm weak? Can you move it?”); and a confrontation task (e.g., “Grab my hand with your left hand”). The question in the confrontation task is asked while the examiner’s hand is held up at eye level in the patient’s right visual field. The patient is then asked, “Did you do it?”) For the present study, a bilateral task (“Lift both arms in the air”) was added (
12).
To explore sensorimotor experience, we modified the follow-up questions to the unilateral and bilateral tasks. After the patient was asked “Did you do it?” or “Are you doing it?,” respectively, we recorded the patient’s verbal responses. If the patient claimed movement by responding “yes,” follow-up questions to characterize the patient’s sensorimotor experience were asked: “You did?”; “Did you feel it?”; “Did you see it?”; “Is your left hand in the same position as the right?” (for the bilateral task). Opportunity was provided for patients to expound on their sensations.
During all phases of the modified Berti Awareness Interview, the patient was observed closely for the following neurological signs: allochiria, displays of intention, and implicit knowledge.
An allochiric response was defined as either attentional (i.e., after the question “Is your left hand weak?”, the patient immediately attends toward his or her right hand with a versive head turn and eye gaze directed toward the right hand, and spontaneous movement of the right hand, while at the same time providing a verbal response to the question about the left hand) or motor (allokinesis) (after the request “Grab my hand with your left hand,” the patient instead does this movement with the right hand and is unaware that he or she has used the wrong hand) (
2).
The presence of implicit knowledge was defined as the following spontaneous movements: After the request “Grab my hand with your left hand,” the patient instead uses the right hand to either grab the examiner’s hand, pulling it toward the left hemispace and forcing the examiner’s hand to touch or nearly touch the limp plegic left hand, or reach with the right hand into the left hemispace and pick up the limp plegic left hand, pulling it toward the examiner’s hand. This right-sided purposeful movement is distinguished in part from allochiria because it spans both hemispaces, whereas allochiric movements remain isolated to the right hemispace.
Displays of intention were defined as an observable display of motor effort after being asked to grab the examiner’s hand, indicated by trunk flexion and displacement during the reach attempt. This is based on the fact that hemiparetic patients making reaching movements employ a compensatory strategy of trunk flexion to achieve their reaching goal (
26,
27).
Statistical Analysis
To analyze relationships among clinical and neuropsychological variables, nonparametric continuous variables were compared by using the Mann-Whitney U test, and categorical variables were compared with Fisher’s exact test.
Lesion Mapping
Results for acute MRI scans were available for 19 patients, and data from CT scans were available for the remaining 10 patients. Lesion borders were manually drawn directly on the individual MRI or CT scans by using MRIcron software (
28). Subsequently, both the anatomical scan and the drawn lesion volume were normalized to a standard brain template with the Clinical Toolbox for SPM, using the Statistical Parametric Mapping version 12 software package (Wellcome Trust Centre for Neuroimaging, London) implemented in MATLAB (MathWorks, Natick, Mass.).
After normalization, all lesions were visually inspected to ensure accuracy of registration into common space. These normalized lesions were then used for voxel-based statistical analysis with NiiStat (
https://www.nitrc.org/projects/niistat). In this analysis, with one dependent variable (allochiria, intention, or implicit behavior) and one independent variable (lesion status), a chi-squared Liebermeister test was performed at each voxel, comparing scores on the dependent variable between patients with and without lesions in that voxel (
29). The resultant z-scores were coded along a corresponding color scale mapped onto the standardized anatomical template (
30).
Results
Demographic characteristics of the study participants are presented in
Table 1. All 29 patients had AHP on first examination. Twenty-two patients were re-examined within or at 7 days; of these, seven (32%) had persistent AHP at 1 week.
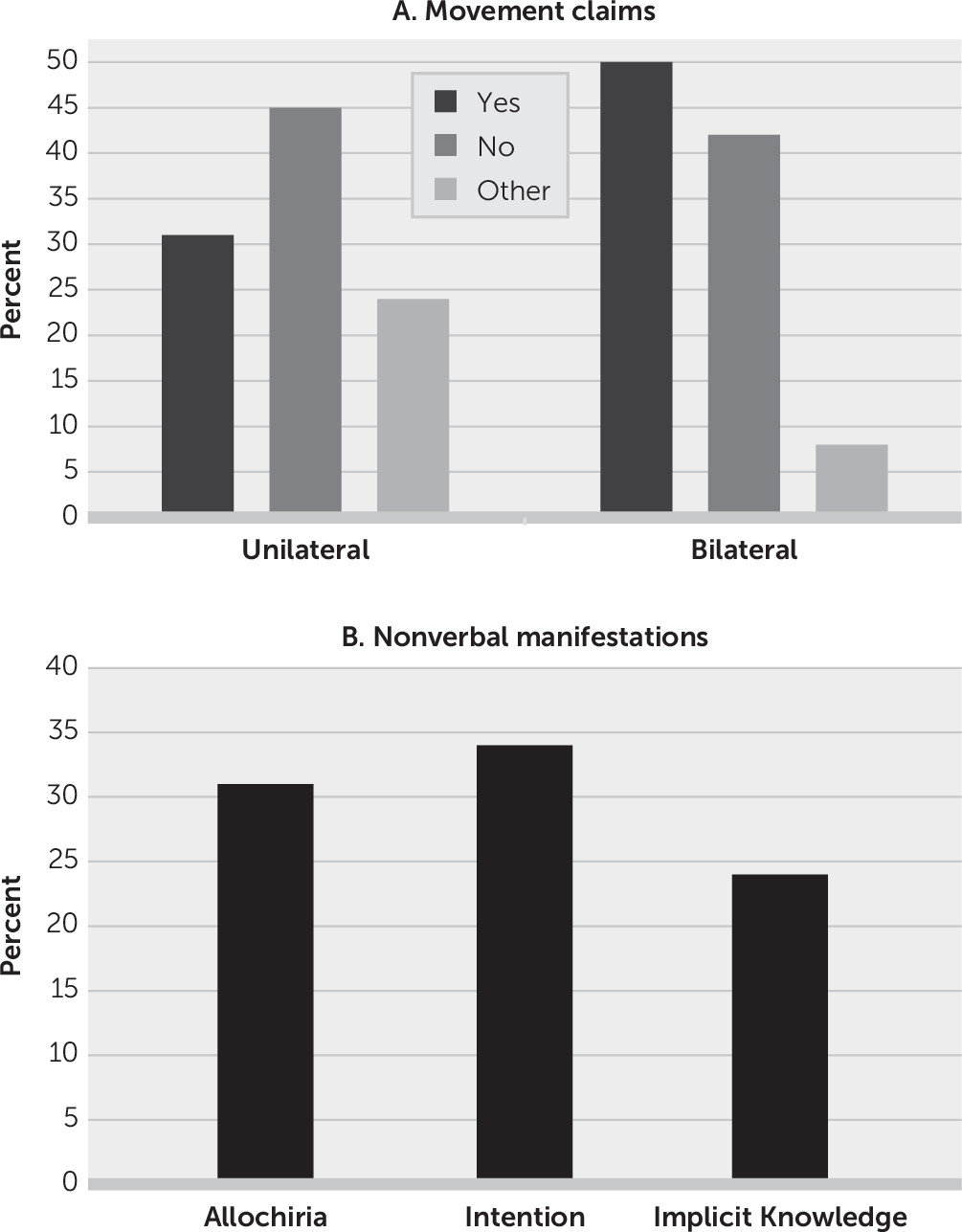
Figure 1 summarizes the results. For the unilateral task (N=29), 13 (45%) patients acknowledged no movement, nine (31%) claimed movement, and seven (24%) could not be categorized (four were silent, two provided irrelevant statements, and one had verbal content only relating to the right side). For the bilateral task (N=24), 10 (42%) patients acknowledged no movement, 12 (50%) claimed movement, and two (8%) were silent.
Nine patients claimed movement during the unilateral task. After being challenged, two of the nine recanted. Two patients claimed kinesthetic details (one patient claimed that he saw and felt movement, while another provided a verbal description of her kinesthetic sense of movement and said that she had some volitional control of it). One patient said that she felt her limb move but was confused about when the movement occurred (on the day of the task or on the day before the task); one thought that she moved her limb but was not certain and felt that she did not see it; one stated “I will” (in response to the question “You did?”); one was silent (when asked follow-up questions); and one was not asked any follow-up questions.
Twelve patients claimed left-arm movement during the bilateral task. Nine were asked follow-up questions. One such question was “Is your left hand in the same position as the right?” Six (66%) of the nine patients answered “yes,” one recanted, and two were silent.
On follow-up examination, 15 of 22 (68%) patients explicitly acknowledged left hemiplegia, and the remaining seven still had AHP. Of the 15 patients with explicit awareness of hemiplegia, four (26%) still claimed movement (both the unilateral and bilateral tasks, N=2; bilateral task only, N=2). Ten patients who claimed movement (unilateral or bilateral task) were examined with the task again on a subsequent day; of these, six (60%) gave an answer that was different from the previous one.
The nonverbal manifestations observed on initial and subsequent examinations yielded the following (
Figure 1): allochiria in 31% (N=9/29) of patients, signs of implicit knowledge in 24% (N=7/29), and intact intention in 34% (N=10/29).
The relationships among clinical and neuropsychological variables were as follows. Twenty-four patients were tested for the presence of movement claims in the unilateral task and then the bilateral task within the same session: the movement claims were not significantly correlated with each other (Fisher’s exact, p=0.371). NIH Stroke Scale scores, neglect severity, allochiria, implicit behavior, intention display, claims of unilateral movement, and asomatognosia were not associated with the persistence of explicit denial of hemiplegia at 1 week. The presence of movement claims during the bilateral task was associated with explicit acknowledgment of hemiplegia at 1 week.
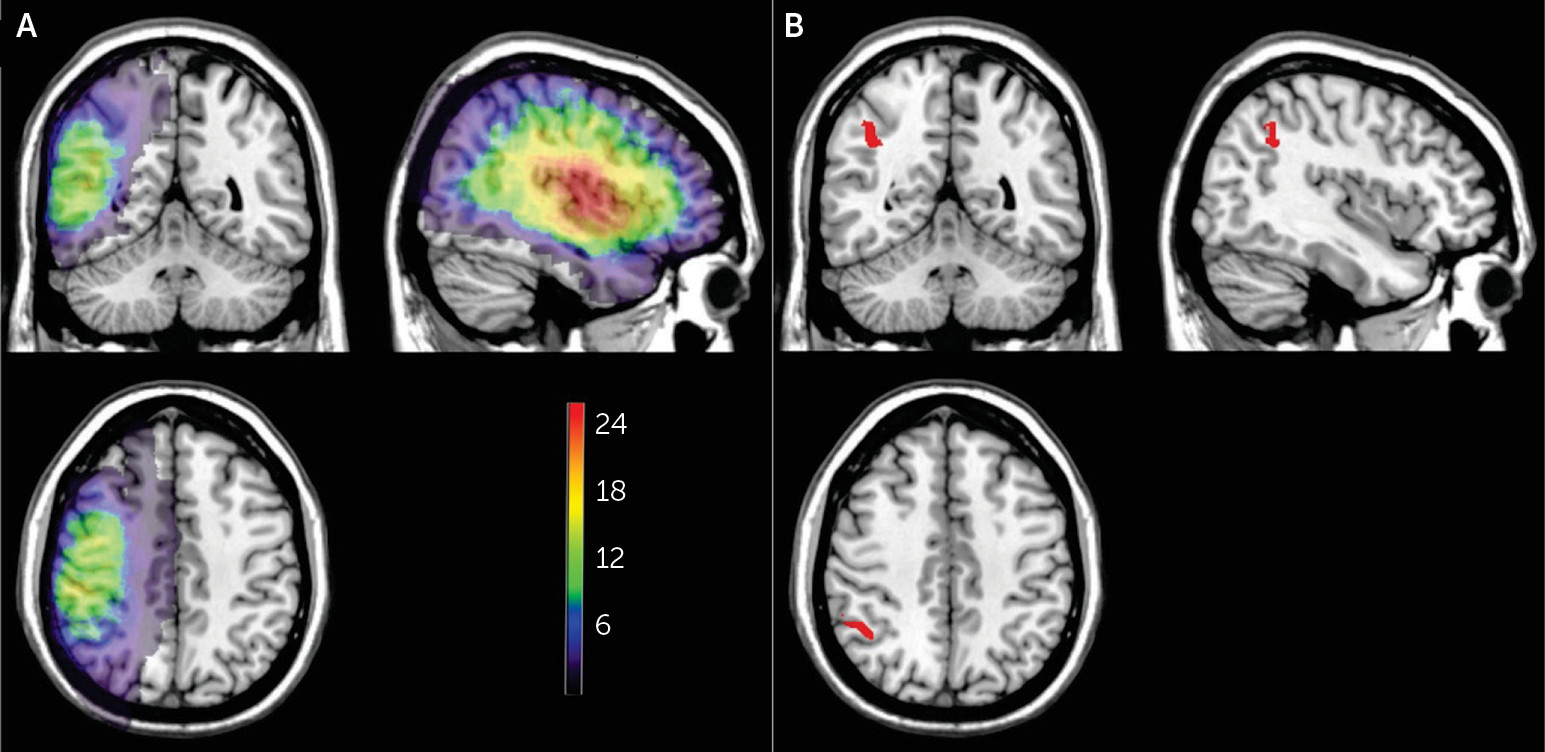
Lesion density plots for all 29 patients are shown in
Figure 2A. Brain regions significantly associated with allochiria are shown in
Figure 2B (on the basis of 28 patients, because of a skull defect in one patient). These 486 voxels survived to the statistical threshold with a p value of 0.05 (critical z-value, 4.3, false discovery rate-corrected for multiple comparison). The largest concentration was located in the inferior parietal lobe, most specifically Brodmann’s area 40. No voxels survived to reach significance with the variables of intention or implicit behavior.
Discussion
The aim of this study was to explore what anosognosic patients experience when attempting to move a plegic limb and their directly observable behavior during movement attempts. Our results demonstrate that movement claims are idiosyncratic and task dependent, lack internal consistency within individual patients, and can dissociate from explicit denial, as several patients made movement claims even after they began to explicitly acknowledge hemiplegia. We found AHP to be a transient disorder, with symptoms in 68% of patients resolving by 1 week. Careful inspection of movement attempts showed that observable behavior related to AHP was displayed in the form of allochiria in 31% of patients, signs of implicit knowledge of weakness in 24%, and intact intention in 34%.
Allochiria was associated with damage to the inferior parietal lobe. Signs of intact implicit knowledge and intention were not associated with any particular damage. Movement claims lacked internal consistency and thus could not be subject to lesion analysis.
These results highlight that heterogeneity, phenomenological complexity, and transience are hallmarks of the disorder. At a clinical level, our results advance clinical AHP assessment by showing that assessment of performance, rather than just verbal response, during Berti’s task efficiently adds important dimensions not captured by typical severity scales. On a theoretical level, these results provide useful constraints on pathogenic models and do not lend support to unitary models that propose, for example, that illusions of movement or impaired intention form the basis for the disorder. Rather, the heterogeneous clinical expression of AHP we describe suggests that the genesis of the disorder is etiologically diverse.
Sensorimotor
We found that explicit awareness of a movement failure is common in AHP. On the unilateral task, most patients did not claim movement (69%); 45% responded “no,” and 24% were silent or provided a verbal account with irrelevant content. Of the nine (31%) patients who claimed movement, four stood by their claim and reported at least some kinesthetic or postural experiences in support of their claim, and two (22%) recanted their claim. On the bilateral task, 12 patients (50%) claimed movement; of these, nine (66%) stood by their claim.
Movement claims were not correlated with each other within individuals (Fisher’s exact, p=0.371); that is, a patient’s claim of movement during the unilateral task was not associated with a claim of movement during the bilateral task. This lack of correlation indicates that movement claims per se lack internal consistency within individuals. Moreover, upon follow-up testing, movement claims were dissociated from explicit denial, because several patients made claims even after they began to explicitly acknowledge hemiplegia (N=4/15).
These results are difficult to reconcile with motor efference theory as the primary basis of AHP. If a kinesthetic percept underlies a patient’s judgment that he or she is not weak, then one might expect that a higher percentage of patients would claim movement, that their reports would be stable from one movement attempt to another, or that movement claims would not persist after explicit recognition of weakness returns. Moreover, movement claims generally are not accompanied by robust verbal description. An alternative viewpoint from motor efference is that movement attempts need not produce a kinesthetic percept to result in a movement claim; rather, a movement claim would be a classic example of a confabulation generated by the left hemisphere in the setting of inappropriate input received from a damaged right hemisphere—a framework supported by several decades worth of split-brain research (
31).
What emerges is a complex picture. In the moment during the task, patients may be aware of a movement failure yet go on to continue to deny hemiplegia. Conversely, patients may be unaware of movement failure, claiming movement, yet have explicit awareness of hemiplegia. Their momentary experience of movement attempts and the consequences of these attempts do not appear to be tethered to denial as a whole. Marcel et al. (
32) observed similar behavior and suggested that many patients who experience anosognosia do not integrate these momentary experiences in their long-term knowledge about their bodies.
The heterogeneous and dynamic sensorimotor experiences we report suggest that AHP is a multicomponent syndrome. Different combinations of deficits (impaired body perception, multisensory integration, learning and memory, and spatial neglect) at varying levels of severity combine to produce the disorder and may vary from patient to patient and even moment to moment in a single patient.
Allochiria
We found that 31% of patients displayed an allochiric response. Two types of responses were observed: attentional (after being asked about the strength of his or her left hand, the patient immediately attended with a versive head turn and gaze directed toward the right hand and spontaneously moved the right hand) and motor (when asked to move the left hand, the patient moved the right instead). Simultaneous to these nonverbal responses, the patient provided a verbal report in response to the question about the left hand, unaware that he or she was attending to the opposite side.
Sensory allochiria, in which a stimulus is reported to have occurred on the opposite side of the body or space, has been reported for tactile, visual, and olfactory senses (
2,
33). Within the motor domain, a case of allochiria (also referred to as allokinesia) was first reported in the literature in 1941 regarding a patient with left hemiplegia from a right-hemisphere stroke (
34). Since that time, allochiria has been presented in clinical neurology textbooks as an observable manifestation of neglect (
1,
2,
35). Stone et al. (
36) found sensory or motor allochiria present in up to 57% of patients with right-hemisphere damage examined 2–3 days after onset. Interestingly, the Stone et al. study is the only incidence study of allochiria. To our knowledge, its natural history, underlying neuroanatomy, and relationship with AHP have never been examined.
One-third of the patients in the present study had allochiria, and lesion analysis showed that it was associated with damage to the inferior parietal lobe. These clinical-anatomical results substantiate allochiria as a meaningful variable in the pursuit of the pathogenesis of AHP. Human and primate studies point to this parietal region as critical for spatial cognition, sensorimotor integration, and movement planning (
37). These patients with allochiria did not have more severe visual neglect than the other patients on the letter A cancellation task. Yet, they had a unique spatial bias that distorted their ability to verbally reason (about their left arm) and plan movements (of their left arm) that restricted them toward only the right side of space.
Intention
The will to move and the subsequent proprioceptive sensations are closely linked (
5). The role intention plays in AHP has influenced theoretical models; however, clinical studies on this relationship have been limited to single-patient studies with conflicting results, some of which have demonstrated preserved physiologic measures of intention, while others have not (
38,
39). To our knowledge, our study is the first to examine the prevalence of preserved intention in a cohort of patients with AHP. Our results help to explain the inconsistency of previous reports: there is heterogeneity.
We found that 34% of patients with AHP exhibited physical signs of intention as measured by observable truncal flexion during a reaching task. This may be an underestimation, because trunk muscle recruitment below the level of detection from visual observation, but present on EMG or kinematics, might have an even higher prevalence. These results do not lend support to the feedforward hypothesis of AHP as the primary basis for the disorder.
Implicit Knowledge
Implicit knowledge of deficit has received growing attention in recent years. By using batteries to observe the movement strategy patients with AHP employ to perform two-handed tasks, Cocchini et al. (
17) demonstrated that 25% of patients with right-hemisphere damage exhibit implicit knowledge of weakness. Subtler forms of implicit knowledge have been shown in measuring response times to deficit-related words (
40).
The implicit responses we observed were not subtle, and we found them in 24% of AHP patients. The dissociated behavior was striking to observe. While verbally denying weakness, a patient would use his or her normal hand to either pull the examiner’s hand or lift and pull the plegic left hand toward the examiner’s hand in an attempt to accomplish the goal of “grab my hand with your left hand.” These patients denied paralysis, yet they employed a strategy that clearly demonstrated some implicit knowledge of motor impairment.
To our knowledge, this finding has not been previously reported, and the reason for this is not entirely clear. Surely, countless clinicians and researchers have observed this phenomenon in AHP. However, the standard AHP scales do not provide a way to capture such observations. Moreover, certain movement requests, such as Berti’s task, may be more apt to elicit the response than other requests. For example, after a bilateral request, the intact right hand is occupied and not free to provide help to the left. These issues might have led to the underreporting of the phenomenon. Whatever the case may be, our findings lend support to the idea that assessment of performance, rather than just verbal response, may tap into different aspects of the disorder, allowing the fractures of awareness to become more visible.
Timing
Our study reinforces what the few longitudinal AHP studies have found: AHP is a transient disorder (
41,
42). This natural history of spontaneous recovery from AHP is similar to that of vertigo from cerebellar stroke, and this has important implications for the study of AHP. It suggests that, just as in vertigo, acute phenomenology is the optimal guide for constraining the pathogenic framework of AHP, whereas the study of persistent AHP (similar to persistent vertigo) may help guide an understanding of the failure of typical compensatory mechanisms.