Pediatric brain tumors are the most common form of solid neoplasms in children and the most common cause of cancer death in children under age 14 in the United States (
1,
2). Unfortunately, brain tumors usually do not have a pathognomonic clinical presentation. The initial presenting symptoms might mimic other more common conditions, such as migraine or gastroenteritis (
3). This is particularly significant in younger patients for whom the neurological examination may be unreliable in identifying signs and symptoms revealing acute neurological decline. Symptoms may be subtle but could include headaches, lethargy, motor or sensory deficits, and signs of elevated intracranial pressure, such as nausea, vomiting, or visual disturbance.
Conventional wisdom is that pediatric brain tumors do not frequently present with psychiatric symptoms, such as mood swings or irritability. However, a recent review reported that behavioral and psychological symptoms were present in up to 57% of cases of CNS tumors among pediatric patients (
4). These symptoms may include apathy, depression, mania, personality change, and eating disorders and may occur prior to the diagnosis or early during the clinical course of illness (
5). Still, psychotic symptoms have not been commonly reported in the context of brain tumors, particularly in children.
Here, we present a case of this uncommon presentation: a brain tumor with associated psychotic symptoms in a school-aged child. An incidentally identified left-temporal lobe mass appeared to be associated with progressive psychosis and mood disorder symptoms. Despite aggressive pharmacologic treatment, psychiatric symptoms progressed. After several years of clinical observation, the psychiatric symptoms proved refractory to medications. Although radiologic progression had not clearly occurred, the decision was made to perform surgical resection of the mass, which was identified as a grade II ependymoma, and the child’s psychotic symptoms rapidly abated postoperatively.
Case Report
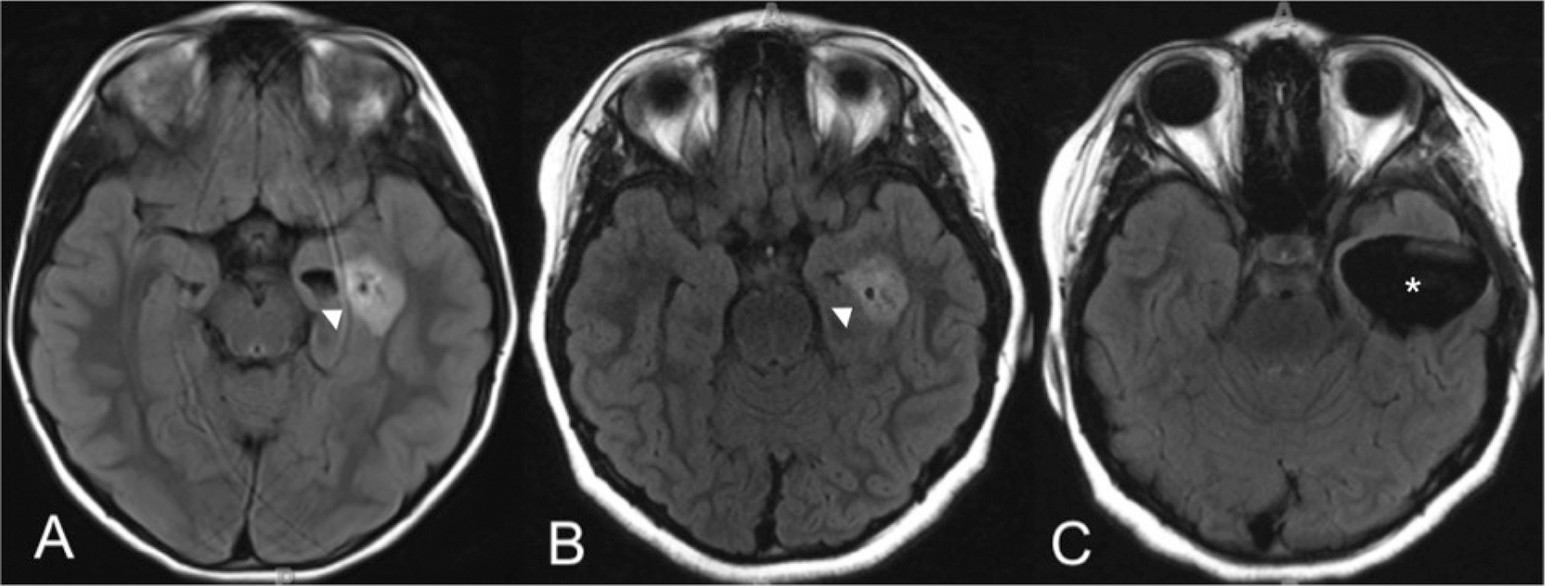
A pediatric patient initially presented at age 6 with a long-standing history of difficult-to-treat gastroesophageal reflux disease with aspiration as well as moderately persistent asthma. Prior to a planned Nissen fundoplication, the patient underwent brain imaging to rule out intracranial lesions associated with persistent nausea and reflux. MRI revealed a left-temporal heterogeneously enhancing mass (2.2×1.6×1.3 cm), with little surrounding edema or mass effect. At this juncture, given the lack of neurological clinical findings and the low likelihood of the mass contributing to her gastrointestinal symptoms, the decision was made to conduct clinical follow-up with serial, repeated MRI. Her gastrointestinal symptoms continued to be managed medically.
Initial examination revealed a healthy appearing, well-nourished, well-developed right-handed female in no acute distress. The child was without developmental delay and had full range and stable affect. No focal motor or sensory deficits were found on neurological examination. Her reflexes were brisk and symmetric. No seizure activity was identified, and other medical history and review of systems were unremarkable, except for presenting symptoms and mild asthma. Regarding the mass, the patient was followed clinically and with serial MRI over a 3-year period. Although the mass size slightly fluctuated (actually getting smaller in the transverse dimension, 2.2×1.8×1.5 cm), the patient did not show signs of disease progression (
Figure 1). Spinal imaging was negative for the presence of metastases.
Shortly after initial presentation, therapy with a psychotherapist was initiated for mild anxiety and behavioral concerns. The child experienced brief, intermittent crying spells and had one instance of suicidal ideation associated with a social stressor. Mood concerns were successfully reduced through implementation of parent-management training strategies; however, the family continued ongoing psychotherapy to closely monitor symptoms. Approximately 1.5 years after the initial identification of the mass, the patient had a recurrence of depressed mood, vague suicidal ideation, and gradually increasing auditory and visual hallucinations. A complete neurological work-up did not reveal causation for changes in symptoms. Three routine EEGs were negative for epileptiform findings; however, intermittent slow activity maximal over the left-temporal lobe was noted.
Over the next year, the child had a worsening course that required three visits to the emergency department, a brief hospital stay, and more intensive outpatient psychiatric treatment. Her symptoms included worsening anxiety bordering on panic attacks, intermittent depressed mood, suicidal ideation, and continued auditory and visual hallucinations. The auditory hallucinations were unrelated to mood at times and included prominent experiences of the “devil” communicating with her and telling her to harm herself or others, although she could resist the hallucinations, because “she knew what it was.”
She was considered to have a primary mood disorder and was treated with escitalopram to target depressive and anxiety symptoms. Additionally, aripiprazole was later added to address her intrusive auditory hallucinations. Aripiprazole, at a dosage of 5 mg daily, was initially effective in reducing the hallucinations, but the efficacy gradually waned despite dose adjustments. In addition, the patient experienced significant side effects, including a 23-kg weight gain over a 2-year time period (increasing weight from the 75th percentile to the 98th percentile). The severe obesity worsened her underlying asthma and gastrointestinal symptoms. She continued psychotherapy concurrent with her psychiatric treatment but worsened academically and socially and gradually became more isolated from her family, with limited activities beyond engaging in media consumption. After extensive consultation with the psychiatric, oncologic, and neurosurgical teams, the decision was made to remove the tumor surgically, because it was considered to be a potential etiology for the psychosis and mood disorder.
Under general anesthesia, a standard temporal craniotomy was performed with image guidance. The patient’s brain was not found to be edematous. A medial temporal gyrus corticectomy was performed. The tumor was tough in consistency and mostly avascular. The mass abutted against the lateral ventricle and head of the hippocampus and was connected vascularly from the choroid plexus in the lateral ventricle on the superior aspect of the temporal horn. On the medial surface, it appeared that the tumor was attached to the ependymal surface overlying the head of the hippocampus. Through the resection process, the ependymal surface of the hippocampal head was uncovered to the extent that the gray matter within was well visualized, although there was still white matter border surrounding the tumor resection cavity. Intraoperatively, it was apparent that the amount of neoplastic tissue was more significant than initially appreciated by radiographic findings. Gross total resection was achieved.
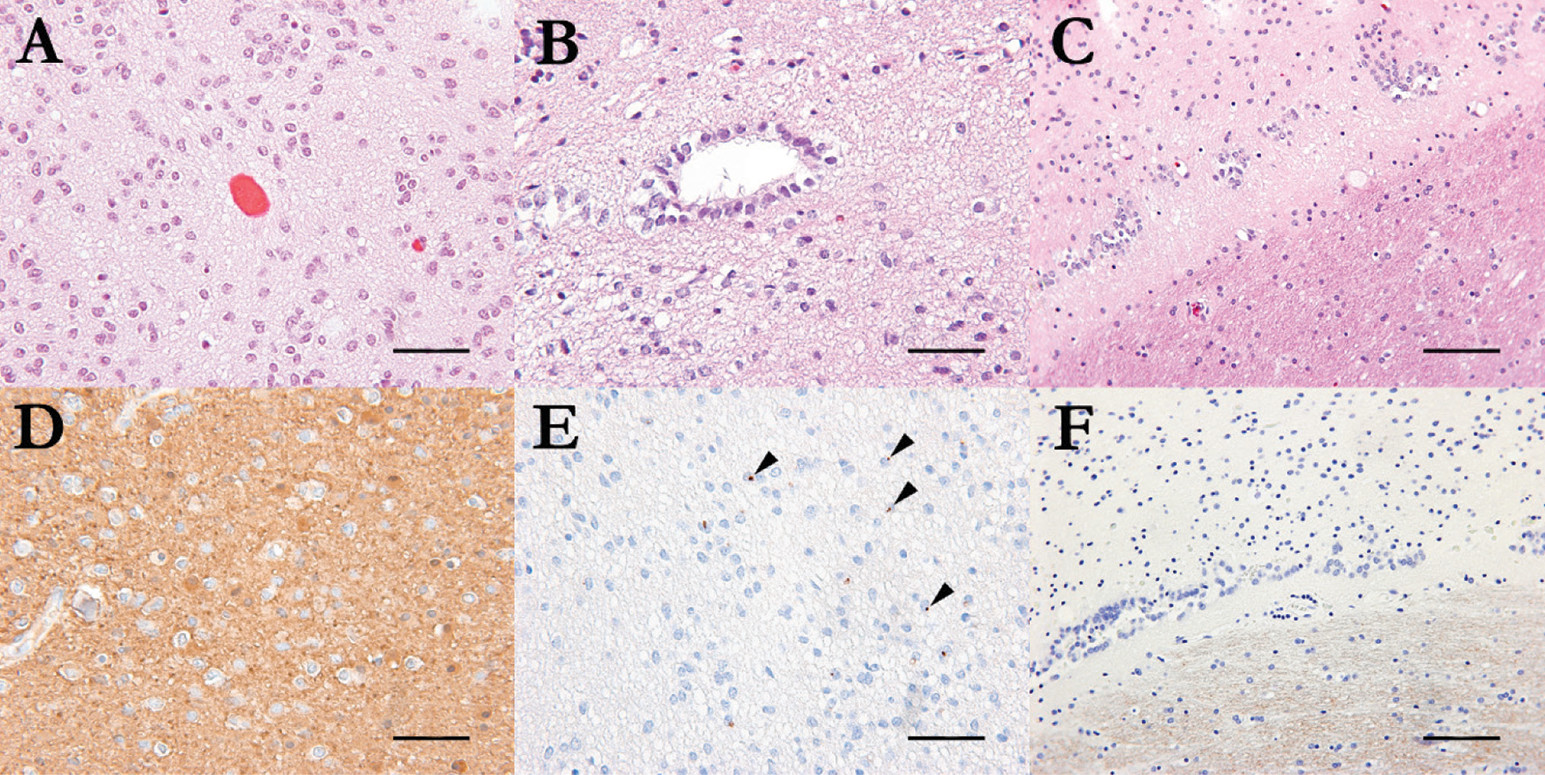
Histopathologic examination revealed a low-grade tumor compatible with ependymoma (grade II, using the World Health Organization tumor grading system), with a Ki67 proliferation index <1%. Immunohistochemical staining demonstrated
GFAP and
EMA expression in neoplastic cells. Schistosoma mansoni proteases SM31 immunostaining showed no significant infiltration by neoplastic cells (
Figure 2). P53, OLIG2, and mutant IDH1 proteins were negative.
The immediate postoperative course was uneventful, and the patient’s neurological examination was unchanged. In addition, her ophthalmologic and audiologic examinations were normal. She continued outpatient psychiatric treatment following the resection of her tumor. Over the next year, the child’s mood and anxiety symptoms significantly improved, and there were no further instances of hallucinations or suicidal or homicidal ideations. Neuropsychological testing was completed 3 months after surgery and indicated a decline of 18 points in her full-scale IQ and possibly worsened memory function. Despite these difficulties, she returned to school full-time, with satisfactory academic achievement. Two years following her surgery, the patient remained free from hallucinations, was tapered off all of her psychiatric medications, lost weight, and had no evidence of recurrence on MRI.
Discussion
Ependymoma is the third most common brain tumor in children after medulloblastoma and astrocytoma. Factors such as increasing age, spinal location, and gross-total resection are reported to be associated with improved survival (
6). Recent studies have identified nine molecular groups of ependymoma, three from each principal anatomical location of the neuraxis, including the supratentorial, posterior fossa, and spinal compartments (
7). Molecular classification of ependymoma will likely play a major role in the stratification of patients for adjuvant therapy. Although the existing literature suggests that it may be unusual with pediatric patients to expect psychotic symptoms to result from a brain tumor, the above case suggests otherwise. Specific tumors are not consistently associated with specific psychiatric symptoms; however, some cases suggest that the location of the tumor may play a role. Kugaya et al
. (
8) reported a case of ependymal cyst resection from the posterior aspect of the left-lateral ventricle, which led to resolution of depressed mood, depersonalization, and suicidal ideation in the patient.
In the above case, the discordance between the radiographic and clinical findings was remarkable, even though there was some uncertainty about specific connectivity or physiology that could explain the patient’s symptoms. However, the tumor itself abutted against the hippocampus and was likely to have caused mass effect, albeit subtle. The resection process itself required significant exposure of the hippocampal head. Extension of the tumor disrupting the integrity of the hippocampal space could plausibly be expected to affect function, if not by mass effect, then perhaps by altering physiologic stability. Electrographically, even though epileptiform findings were not present, slowing was intermittent and prominent over the left-temporal lobe, also suggesting a focal cerebral disturbance.
Ultimately, this case strongly implicates the temporal lobe as relevant in symptoms of depression as well as hallucinations. The temporal lobe’s role in long-term memory is also implicated in this case and may represent an example of potentially negative consequences of surgical resection in this location (
2,
9). Neural circuitry involving the temporal lobe is known to play a role in affective disorders and is also suspected in psychosis (
10,
11). Any type of pathology affecting the temporal lobe may then be intuitively expected to have an effect on such psychiatric symptoms. In pediatrics, mood disorders appear to be more common for seizure localization in the temporal lobe, especially if the seizures are refractory to medical treatment (
12,
13).
Whether this case reflects unique roles of the tumor or the role of the location of the tumor is difficult to establish. Primarily, this case highlights the challenging aspects of managing young patients who have suspected tumors that are incidentally diagnosed. Although a CNS mass uncommonly underlies newly presenting psychiatric symptoms among children, in our patient, psychiatric symptoms were notably refractory to pharmacologic and psychotherapeutic interventions. This ultimately compelled the treatment team to pursue surgery despite apparently stable MRI. Although still speculative, the fact that the tumor progressed well beyond what could be identified radiographically suggests that the psychiatric symptoms likely resulted from the tumor effect.
Our patient’s rapid postoperative improvement perhaps even more convincingly demonstrated that the tumor was the etiology of her symptoms. Despite memory problems, her overall outcome has positively affected her quality of life and that of her family. This case highlights the importance of keeping brain tumors on the differential diagnosis for children who present with new psychiatric symptoms, especially psychotic symptoms, which can be associated with temporal lobe involvement. Additionally, it is one of the few cases in the pediatric literature, to our knowledge, to directly demonstrate that surgical management to remove a previously unrecognized brain tumor may be a successful treatment for serious medically refractory psychiatric symptoms.