Assessing mental health difficulties among people with neurodegenerative disorders can be clinically challenging, because communication problems, cognitive deficits, and reduced self-awareness are common. Clinicians may assess via clinical interview, patient self-report, clinician observations of behavior, or consultation with an informant (usually a partner, relative, or other caregiver). Informant consultation is commonly used with patient groups when accuracy of self-report might be compromised. However, little research has explored whether the presence of an informant may affect the assessment process and outcomes. For clinicians and researchers to accurately assess mental health among vulnerable groups and provide appropriate support and interventions to maintain well-being, it is important for this to be investigated.
A key neurodegenerative disorder in which emotional difficulties can be difficult to detect and quantify is Huntington’s disease (HD). HD is an autosomal dominant neurodegenerative disease characterized by progressive regional brain atrophy and decline in motor, behavioral, emotional, and cognitive well-being (
1–
3). It affects approximately six people per 100,000 across Europe, North America, and Australia, with lower documented prevalence in Asia (
4). Common emotional and behavioral difficulties among persons with HD include depression, anxiety, irritability-anger, aggression, perseveration, disinhibition, and apathy (
5–
14). These symptoms are often accompanied by anosognosia (lack of insight or awareness) or symptom denial, typically increasing with disease duration and severity (
12,
15–
17).
Given the complexities of motor and cognitive neurodegeneration, accurate diagnosis of mental health difficulties among individuals with HD can be particularly difficult. This may be reflected in highly varied prevalence rates; anxiety estimates range from 13% to 71% (
18), and depression estimates from 15% to 69% (
8,
19). Such variability has been attributed to the wide variety of assessment tools (
8,
18,
20), but standard clinical measures of mental health may also be unsuitable for persons with HD because of confounding physical aspects of neurodegeneration. For example, sleep and appetite are DSM-5 diagnostic indicators for depression, but they are commonly disrupted in HD and thus are less valid indicators of low mood. Therefore, self-report is typically used alongside the clinical interview to identify co-occurring mental health difficulties (
21). However, even though some studies have reported reasonable agreement between self-reports of HD patients and reports of their informants regarding apathy (
5,
22,
23) and irritability (
22), other studies have demonstrated that discrepancies between patient and informant ratings typically increase over time (
15–
17,
24–
26). Such discrepancies may be caused by deteriorating insight into symptoms (
27); loss of motor skills required to complete paper-based assessments; and worsening memory, which hinders the retention and retrieval of relevant information (
21,
28,
29).
Consequently, the accuracy of self-report may be compromised as the disease progresses, cognition declines, and symptom burden rises (
30), meaning increasing reliance on informant report. Informants offer additional important information. For example, Underwood et al. (
31) found no association between self-rated pain and irritability, whereas informant ratings of pain were significantly associated with irritability. Similarly, Duff et al. (
7) found no predictive validity for self-report of executive dysfunction regarding later motor impairment, whereas informant report had predictive validity, even 10 years before the manifest stage. Further, Simpson et al. (
14) found that compared with their caregivers, individuals with HD reported lower symptom frequency (often missing symptoms altogether, which caregivers reported) and overestimated their abilities; in addition, patient and caregiver judgments about which symptoms were most impactful were relatively disparate. Although these findings do not imply that either patients or caregivers were “wrong,” they do highlight the discordance and potential threat to validity of assessment processes if such discrepancies are not taken into account. The discrepancies can also be important sources of clinical information, leading some researchers to recommend use of measures, such as the Neuropsychiatric Inventory and the short-form Problem Behaviors Assessment for HD (PBA-s) (
29,
30), that integrate patient, informant, and clinician report as well as interviewer observations. This approach to assessment helps to ensure that important patient-centered information crucial to effective care and understanding of the individual is retained but that it is combined with information from informants unaffected by HD symptoms.
It is crucial to consider the format in which these perspectives are sought and integrated, because this may affect reliability and validity of outcomes. Typical approaches in neuropsychology include patient report, informant report (gathered separately), and joint interviews. Crucially, dynamics between patient and informant in joint interviews can powerfully influence reporting of symptoms and how the interviewer interprets and rates these responses. For example, informant presence might help to correct self-reports of patients who underreport symptom severity or frequency because of impaired insight or memory. However, patients might also minimize their symptoms to avoid distressing informants, thus decreasing accuracy, and the informant may even collude in this minimization to avoid conflict or because he or she is in denial (especially those at genetic risk themselves). An informant might also underestimate symptoms that are difficult to identify from observation (e.g., low mood) if the person with HD chooses not to—or has become unable to—communicate his or her experience explicitly, which may be associated with loss of perceived “depth” in conversation as HD progresses (
32). Finally, there may be natural differences of opinion within relationships, whether neurodegenerative disease is involved or not, and especially regarding negatively perceived qualities such as irritability or apathy. These complex potential effects of informant presence on the validity and reliability of mental health measures in neurodegeneration have not been explored, and identifying discrepancies between informant-present and informant-absent symptom reporting is thus a high clinical priority.
This study aimed to explore differences in ratings of emotional symptoms between participants who attend a clinical interview with and without an informant, with the intention of shedding light on crucial unexamined factors in the assessment process. To enable evaluation of both the influence of informant-interviewee dynamics and disease-related variables, these differences were examined in manifest and premanifest HD participants and in persons in a control group (genotype-negative individuals and relatives without genetic risk of HD).
Methods
Participants
All participants were engaged in Enroll-HD. Enroll-HD is a global clinical research platform designed to facilitate clinical research in HD. Core data sets are collected annually from all research participants as part of this multicenter longitudinal observational study. Data are monitored for quality and accuracy by using a risk-based monitoring approach. All sites are required to obtain and maintain local ethical approval.
First-visit data for 8,714 Enroll-HD participants were initially examined for completeness. Those who lacked data for all variables reported in this study were excluded, for a total of 7,914 respondents: Europe, N=4,254; Northern America, N=3,301; Australasia, N=312; and Latin America, N=47. Of these, 4,109 respondents (2,028 males and 2,081 females, with a mean age of 52.60 [SD=12.5]) were manifest for HD, 1,790 respondents (705 males and 1,085 females, with a mean age of 40.43, [SD=11.9]) were verified premanifest HD carriers, 1,041 respondents (350 males and 691 females, with a mean age of 43.01 [SD=14.3]) were genotype negative (i.e., individuals who have a first- or second-degree relative who is a carrier, but who are known not to carry the HD expansion mutation themselves), and 974 respondents (433 males and 541 females, with a mean age of 54.19, [SD=12.4]) who were family members with no genetic risk of HD (e.g., spouses of individuals with HD). Among the manifest HD sample, 6.4% (N=265) had a Total Functional Capacity (TFC; a measure of overall abilities and functioning) score of 2 and below (indicating stages IV and V of HD).
This study examined disparity in PBA-s subscale scores between persons who attended the assessment without an informant (N=3,881) and those who attended with an informant (N=4,033). Among the 4,033 participants who had a PBA-s assessment with an accompanying informant, 2,584 attended with a spouse or partner, 466 with a parent, 340 with one of their children, 296 with a sibling, 108 with another relative, 103 with a professional care worker, 92 with a friend or neighbor, and 44 with an “other” individual.
Measures
The PBA-s comprises 11 items rating different HD-related behavioral difficulties on severity and frequency of occurrence using 5-point scales, which are multiplied together to generate overall scores for each domain (
10). It is a valid measure with good test-retest reliability (Cohen’s weighted κ=0.94 for severity scores and 0.92 for frequency scores) (
33) and produces a three-factor structure comprising apathy (apathy, perseveration, and disorientation), irritability (irritability and aggression), and affective (low mood, suicidal ideation, and anxiety) factor loadings (
13).
We included a number of control variables in the analysis that are known to be correlates of mental well-being or its reporting. In addition to sex and age, we assessed two disease-related variables: cytosine-adenine-guanine (CAG) score from the highest allele and TFC score from the United HD Rating Scale (
34) to measure disease impact. We also assessed cognitive ability through verbal fluency total score and Symbol Digit Modalities Test (
35); body mass index (BMI); use of recreational drugs (marijuana, heroin, cocaine, ecstasy, gamma-hydroxybutyrate, flunitrazepam, amphetamines, methylphenidate, hallucinogens, inhalants, opium, painkillers, barbiturates, and tranquilizers); alcohol, caffeine, and tobacco use (use of recreational drugs and of alcohol, caffeine, and tobacco was scored as follows: 0=no use, 1=reported use).
Data Analysis
Analysis of variance was used to examine differences in affect, apathy, and irritability outcome scores between participant-only versus informant-present interviews for manifest, premanifest, genotype-negative control group, and family control group. No significant between-group differences were found for the family control group, and thus this group was excluded from subsequent analyses.
Subsequently, multiple regressions were conducted to examine the effect of the participant-only versus informant-present variable (the outcome variable at step 2) on apathy and irritability scores (the outcome variables) after controlling for the aforementioned demographic, disease-related, cognitive, and health variables known to be related to mental health (step 1). A multiple regression was not conducted for affect, because no significant differences occurred for affect scores for the participant-only versus informant-present variable across all subsamples. The subsample sizes were adequate, given that N>874 is suitable for testing multiple regression models using 12 predictor variables with an anticipated small effect size (f
2=0.02, because smaller effect sizes tend to accompany larger samples [
36,
37]), a statistical power level of 0.8, and a probability level of 0.05.
Results
Table 1 shows mean (and SD) scores by subsample, comparing PBA-s affect, irritability, and apathy subscale mean scores according to participant-only and informant-present groupings. For the premanifest, manifest, and genotype-negative subsamples, the mean scores for irritability and apathy were significantly lower for the participant-only group, compared with the informant-present group (with the exception of irritability in the premanifest subsample, which was higher for the participant-only group).
Six multiple regressions were conducted to examine the extent to which these differences between the participant-only group (scored 0) and the informant-present group (scored 1) (step 2) showed incremental validity for predicting irritability and apathy scores (outcome variable) in the premanifest, manifest, and genotype-negative subsamples while controlling for expected other predictors (as described above) (step 1). The variance inflation factors and tolerance factors for the predictor variables were no larger than 3.03 and no smaller than 0.33, respectively. Therefore, they did not contravene the threshold value for a variance inflation factor ≥5 and for tolerance statistics <0.2 that would otherwise suggest collinearity between independent variables (
38).
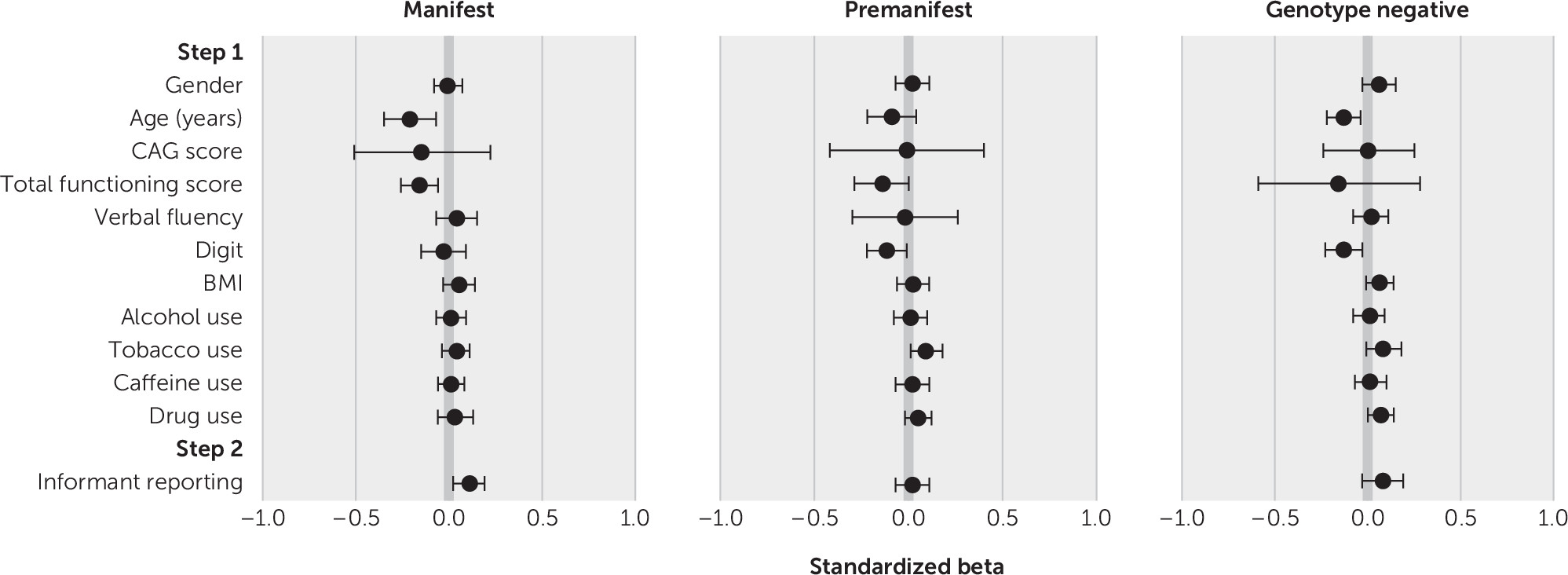
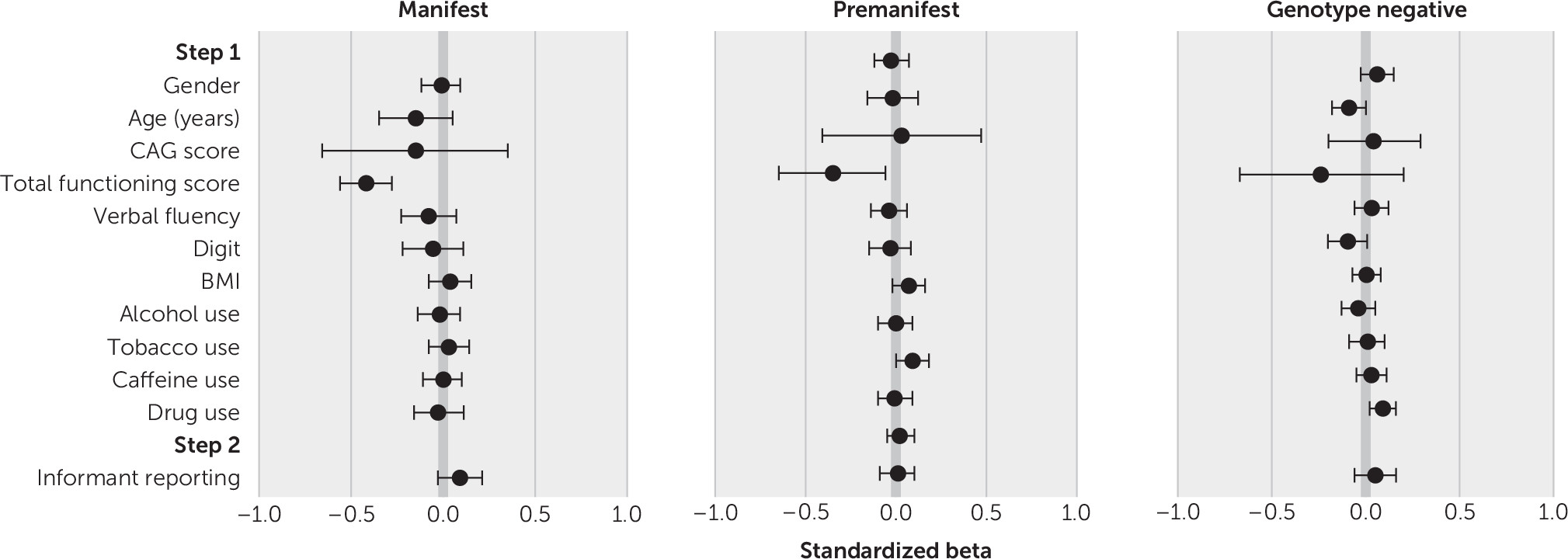
The regression models for irritability and apathy for the manifest, premanifest, and genotype-negative subsamples are presented in
Tables 2 and
3 (forest plots for standardized beta values are shown in
Figures 1 and
2). All models were significant at step 1 for irritability (manifest subsample, F=14.58, r=0.19, adjusted R
2=0.04, p<0.001; premanifest, F=11.10, r=0.25, adjusted R
2=0.06, p<0.001; genotype negative, F=8.06, r=0.28, adjusted R
2=0.07, p<0.001) and apathy (manifest, F=117.87, r=0.49, adjusted R
2=0.24, p<0.001; premanifest, F=31.15, r=0.40, adjusted R
2=0.16, p<0.001; genotype-negative, F=9.08, r=0.30, adjusted R
2=0.08, p<0.001). Multiple step 1 variables were found to predict irritability and apathy. Lower TFC scores predicted higher irritability and apathy for all three subsamples. Lower age predicted higher irritability for all three subsamples and higher apathy for the manifest and genotype-negative subsamples.
Tobacco use predicted higher irritability for all three subsamples and higher apathy for the manifest and premanifest subsamples. Higher BMI predicted higher irritability for the manifest and genotype-negative subsamples and higher apathy for the manifest and premanifest subsamples. Drug use predicted higher irritability in all three subsamples and higher apathy in the genotype-negative group. Lower scores on the Symbol Digit Modalities Test predicted higher irritability in the premanifest and genotype-negative groups and higher apathy among the manifest and genotype-negative subsample. Lower verbal fluency scores predicted higher apathy among the manifest subsample, and being female predicted higher apathy among the genotype-negative subsample. Finally, CAG score predicted higher irritability and apathy in the manifest subsample.
In step 2, informant presence produced a significant change in R2 for all three subsamples for irritability (manifest, ΔF=45.26, ΔR2=0.011, p<0.001; premanifest, ΔF=10.57, ΔR2=0.006, p=0.001; genotype negative, ΔF=6.81, ΔR2=0.006, p=0.009) and a significant change in R2 in the manifest subsample for apathy (ΔF=37.16, ΔR2=0.007, p<0.001).
Discussion
This study revealed important effects of informant presence during the clinical interview. For the manifest group, reports of apathy symptoms of HD diverged between the informant-present and participant-only groups, in line with expectations of lowered insight as the disease progresses. However, findings for affect and irritability were more complex. Outcomes for the three PBA-s domains (apathy, affect, and irritability) are discussed separately.
Apathy (Apathy, Perseveration, and Disorientation)
Lower functioning, as measured by the TFC score, predicted higher apathy across all subsamples. Smoking tobacco also predicted higher apathy in the premanifest and manifest groups, indicating that smoking may require consideration in future research examining the course and variability of apathy in HD.
Informant presence predicted higher apathy outcomes for the manifest subsample, corroborating prior findings that persons with HD become increasingly poor at self-reporting apathy as the disease progresses and executive function is increasingly affected (
22), as well as corroborating broader findings that individuals with HD tend to underreport symptom severity and frequency (
14,
30). However, it is important to note dissenting studies reporting that patient- and informant-rated apathy scores were broadly congruent (at least at some points in the disease process) (
5,
23).
On the basis of this study’s findings, however, informant presence during interviews with individuals with manifest HD appeared to lead to higher apathy scores, compared with patient-only interviews. One explanation is that informants may observe signs of demotivation and apathy in the person with HD, and they are able to correct that person’s self-report if the individual underestimates symptoms in this area. An alternative explanation is that patients with higher apathy were more likely to be accompanied to the assessment by an informant because of problems organizing and motivating themselves to attend. Although this explanation is possible, this study controlled for factors that might also affect ability to attend an interview unaccompanied (e.g., TFC score and cognitive ability), and informant presence was nevertheless found to be significant. Therefore, these findings suggest that with regard to apathy, the addition of an informant perspective is particularly useful and might improve the validity of clinical and research findings.
Affect (Low Mood, Suicidal Ideation, and Anxiety)
Informant presence did not significantly influence affect scores in any of the four subsamples. Two explanations are suggested for this. First, individuals with HD may retain good insight in some domains, in line with previous findings discussed above (
5,
22,
23). However, this explanation is questionable, given this study’s findings showing apparent impaired insight into apathy. An alternative hypothesis is that informant report may be more sensitive than self-report only to symptoms that are observable. For example, loss of motivation and increasing apathy are likely to be evident to caregivers and relatives from behavioral changes. Affect, conversely, is arguably more internalized and is largely dependent on the person with HD expressing emotional distress to the relative or caregiver, which may be physically or cognitively difficult or atypical of his or her personality. In the absence of this communication, and especially in later-stage HD, the informant’s ability to report on affect thus depends on having observed and accurately interpreted externalized signs of distress (e.g., tearfulness). There is also a possibility that common indicators of low mood, such as low energy, limited engagement, and difficulties with motivation, may have been captured by the apathy scores rather than the affect scores. Therefore, the informant’s assessment of the patient’s affect may underestimate the patient’s symptoms, potentially bringing the informant’s interpretation more closely in line with the patient’s self-report and preventing the informant’s input from being a useful independent predictor of affect. Importantly, given that individuals with HD appear to underreport other symptoms, such as apathy (as above) and in general (
14,
30), it is likely that affect was also underreported by the manifest group, but the informants were simply not able to help correct upwards, as they appeared to do for apathy. Importantly, this may mean that health professionals are using underestimates of affect-related symptoms to inform care decisions and clinical recommendations, even when informants are present.
Irritability (Irritability and Aggression)
Various significant predictors emerged for irritability outcomes. Lower social function (as measured by the TFC) consistently predicted higher levels of irritability for all three subsamples (premanifest, manifest, and genotype-negative groups), indicating that factors beyond the disease itself appear to be associated with irritability levels—although the direction of causation is unclear, and irritability may, in fact, influence social functioning. Smoking tobacco predicted higher irritability in the premanifest, manifest, and genotype-negative subsamples, and other substance use predicted higher irritability for the premanifest and genotype-negative subsamples. Lower age also predicted higher irritability for all three subsamples. Finally, higher CAG scores and higher BMI predicted higher irritability in the manifest and genotype-negative subsamples. Although fewer CAG scores have previously been found to be significantly associated with lower irritability in people with HD (
39), the reason for this is unclear and may be a subject for exploration in future work.
Informant presence was a significant positive predictor of irritability scores for the manifest and genotype-negative groups (as noted, this analysis was not carried out for family controls, because no significant between-group differences based on informant presence or absence were identified on initial analysis). For the manifest group, this finding concurs with prior work demonstrating discordance in apparent validity between patient-reported and informant-reported irritability (
31). However, although informant presence was thus expected to significantly predict irritability in the manifest group (as it did for apathy), the reporting of higher irritability for the informant-present manifest group and genotype-negative control group is less easily explained. The finding would suggest that self-report of irritability is generally lower than observer report, irrespective of whether a person has HD. Evidence supporting this includes Chatterjee and colleagues’ (
22) finding that self-report of irritability by individuals with HD diverges most from informant report for those with the most intact cognition, suggesting that people generally assess their own irritability quite differently from how others assess them. This discrepancy in assessment may occur because irritability is a socially undesirable characteristic; alternatively, people may construe their own feelings of annoyance as justified rather than regarding their feelings as “irritability,” because the word “irritability” has connotations of unreasonableness, which, again, is socially undesirable. Therefore, discrepancies between self- and informant-rated irritability may be attributable to factors other than anosognosia.
Additional Considerations
It is important to consider the circumstances of the informant’s presence, because these circumstances may represent an uncontrolled confound beyond the scope of this data set (which does not include information about why an informant attended the interview). For example, informant presence may be dictated by patient apathy (the patient may not attend the appointment without active support), distress or anger (the patient may exhibit extreme emotional responses, believing them to be reasonable), or affect (an anxious patient may decline to attend without the informant for support). Therefore, higher symptom scores might be associated with informant presence without being dictated by it. Alternatively, an informant may already be aware that his or her perception of the patient’s symptoms diverges from the patient’s self-report (e.g., because of communication difficulties or perceived lack of insight) and may feel the need to attend and provide “accurate” information. In this sense, the informant-present sample self-selected on grounds that may engender higher scores on the apathy, affect, or irritability domains or that may make a discrepancy between self-reports and informant reports more probable.
A further concern, which could not be addressed in this retrospective study because of limitations in the available data set, is that the comparative validity of self-ratings and informant ratings could not be established. Therefore, although it was possible to identify discrepancies between informant-present and participant-only ratings, it was not possible to determine whether individuals with HD typically lacked full appreciation of their symptoms (or minimized them, perhaps due to neurological or psychodynamic denial [
17]) or whether the informants tended to overestimate patients’ difficulties. However, as noted in two previous studies (
30,
40), it is important to consider that discrepancies between self-ratings and ratings by others can be in themselves clinically informative and that a patient-centered approach should treat both perspectives as crucial to providing high-quality care.
Conclusions
The findings of this study suggest that incorporating informants into mental health assessments appears particularly important for identifying apathy among individuals with HD. Clinicians should also note potential underreporting of irritability and affect, which is not remediated by incorporating informants.