The ability to track time over a wide range of durations (i.e., milliseconds, seconds, minutes, hours, days, months, years) is essential for optimizing interactions with a constantly changing complex world (
9,
10,
16–
19). Timing is a crucial factor in the sensory-motor processing that generates the intricate, precisely controlled movements required to play a musical instrument, catch a ball, or dance. Accurate timing of complex sequences is required for both generating and understanding language. Timing of multiple potentially salient events over longer periods of time is essential for predicting when and where something is likely to happen, in order to respond quickly when opportunities or dangers arise. The importance of timing and temporal processing in movement disorders is well established (
2). Alterations in aspects of temporal processing and timing have also been identified in many other neuropsychiatric conditions (
1,
20–
24). The therapeutic value of temporally based interventions (e.g., rhythmic cueing, slow rhythmic drumming) has been demonstrated for multiple neuropsychiatric conditions (
25–
28).
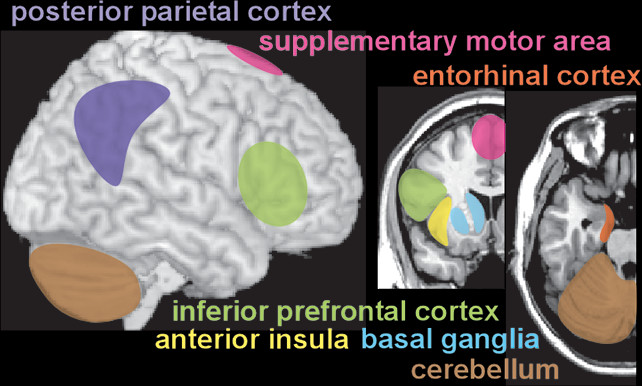
Unlike physical senses, the sense of internal time does not have a specific, dedicated brain region. Temporal perception (inner experience of time) is an abstract concept, constructed within the brain based on available information (external and internal sensory, memory) by a network of areas that likely are specialized for different aspects of timing (
Figure 1 and Cover) (
2,
3,
5–
10). Multiple factors can alter how passage of time is perceived (temporal distortion), indicating the subjective nature of experienced time (
3,
8,
19,
29–
34). In general, rewarding or pleasant circumstances are associated with shortening (time compression) and aversive circumstances (e.g. threatening, painful, stressful, boring) are associated with lengthening (time dilation) of the perceived passage of time.
Memory is fundamental to capturing and using temporal information to guide behavior (
5,
7,
19,
35–
37). It has been known for some time that processing pathways for spatial information (where) and contextual information (what) converge in the medial temporal area. Recent functional MRI (fMRI) studies in healthy adults have implicated the perirhinal cortex and anterolateral entorhinal cortex (ventral stream) in providing temporal order information (when) to hippocampal encoding of memories (
Figure 1 and Cover) (
38–
41). Although the temporal flow of experience is continuous, it is segmented into discrete episodes during encoding (
41–
44). One major hypothesis is that changes in multidimensional context (e.g., turning a corner or stepping through a doorway, beginning a new section in a narrative, changing a behavioral goal) form boundaries. The binding together of events that occur within boundaries creates a single episode. There is evidence that events that occurred in different episodes are perceived as happening farther apart in time (time dilation), and events occurring within an episode are perceived as happening closer in time (time compression) (
36,
42,
44). Increased attention when context changes enough to evoke a boundary may contribute to time dilation across boundaries (
41–
44).
A major approach to identifying the neural underpinnings of temporality and time perception is based on classification of dimensions (taxonomy) (
12,
17,
35,
45). Although details vary, generally agreed-upon dimensions capture duration, pattern, and modality. The critical distinction in duration (i.e., short versus long intervals) is subsecond versus suprasecond timing, as they are believed to have different neural correlates. Patterns of timing are most commonly divided into simple intervals versus complex intervals. An important distinction is whether the timing is of a standard interval or duration (even if repeated) or requires decoding of temporal sequencing (e.g., pattern of events, order of events, location on a mental timeline). A related dimension is whether events are episodic (e.g., isolated intervals) or continuous (e.g., rhythmic, cyclic). Modality is generally divided into sensory (perceptual) versus motor by whether the task-related decision depends on temporal aspects of the sensory input (e.g., judging which stimulus is longer) or developing an internally timed motor response. Another dimension that has been proposed is based on whether use of temporal information is explicitly part of the task (e.g., duration estimate) or can be used implicitly to facilitate performance. Tasks may also differ by whether prospective memory (e.g., participants informed in advance that they will be required to provide duration judgments) or retrospective memory (e.g., participants not informed in advance so they must reconstruct duration information) is required.
Reviews and meta-analyses of functional imaging (fMRI, positron emission tomography [PET]) studies have probed the neural correlates of many aspects of temporal processing in healthy individuals (
1–
11,
13–
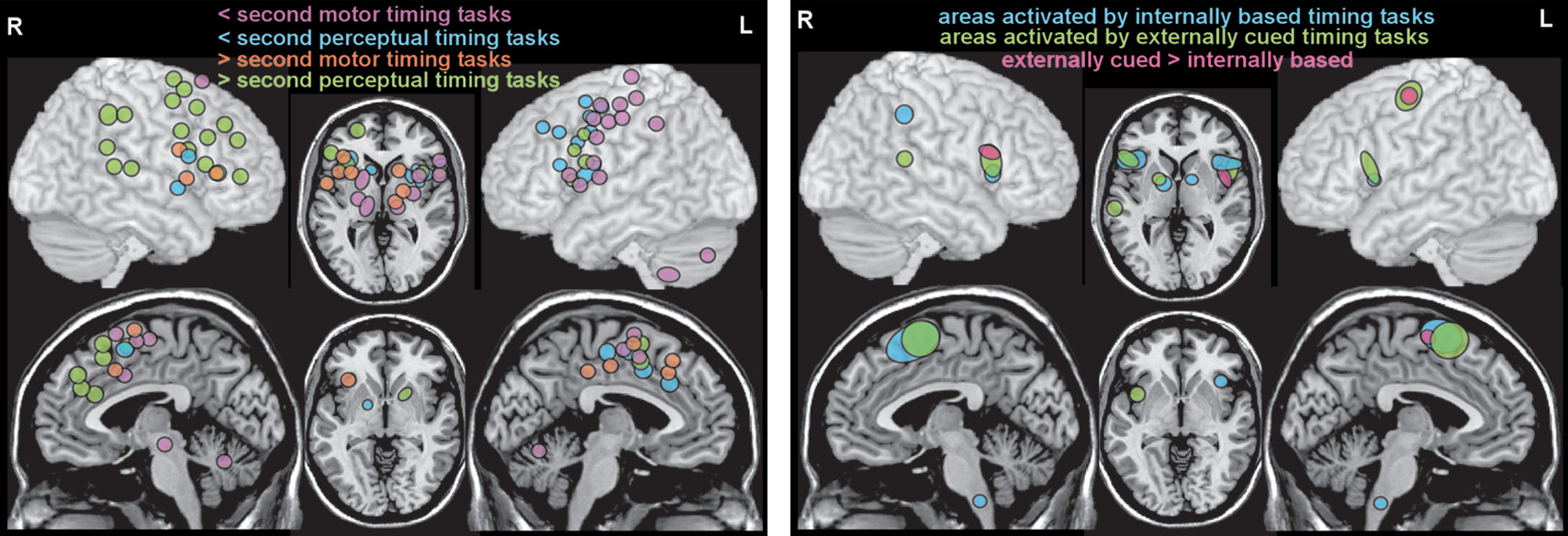
15). Overall, these studies support the presence of a widespread network of cortical (supplementary motor area, premotor cortex, inferior frontal gyrus, anterior insula, inferior parietal cortex, posterior superior temporal gyrus/sulcus) and subcortical (basal ganglia, thalamus, cerebellum) areas that are variably recruited based on specific task parameters and demands (
Figure 1). Meta-analyses of functional imaging studies of temporal processing tasks have identified some dimension-related differences in neural correlates. A recent meta-analysis grouped fMRI and PET studies based on the dimensions of modality (motor, perceptual) and duration (subsecond, suprasecond) (
11). Although the extent and laterality of activations differed across the four conditions, all recruited a relatively similar set of cortical (supplementary motor area, inferior frontal gyrus, anterior insula) and subcortical (basal ganglia) areas (
Figure 2). Activation of the supplementary motor area by all conditions is consistent with its proposed role as a higher level area representing amodal (i.e., regardless of information source) duration. A recent fMRI study in healthy individuals demonstrated a duration-sensitive topographic organization (chronotopic map) for this area (
46). The cerebellum was activated only by the subsecond motor condition, consistent with its role in precise timing (
12). A meta-analysis that compared fMRI studies in which task timing was either provided by external cues (e.g., cued finger tapping, rhythm reproduction or monitoring, temporal prediction) or had to be generated internally (e.g., duration discrimination, interval production or reproduction, interval bisection, self-initiated) also found that both conditions activated a similar network of cortical and subcortical areas (
Figure 2) (
13). A few areas were associated only with internally timed tasks (right: intraparietal sulcus; midline: inferior olive) or externally cued tasks (left: precentral gyrus; right: posterior superior temporal sulcus/gyrus). The contrast analysis identified several areas (bilateral: inferior frontal gyrus/anterior insula; left: supplementary motor area, precentral gyrus) that were activated more in the externally cued tasks (
Figure 2). Overall, these results support some dimension-related differences but not fully dissociable networks (
11,
13).
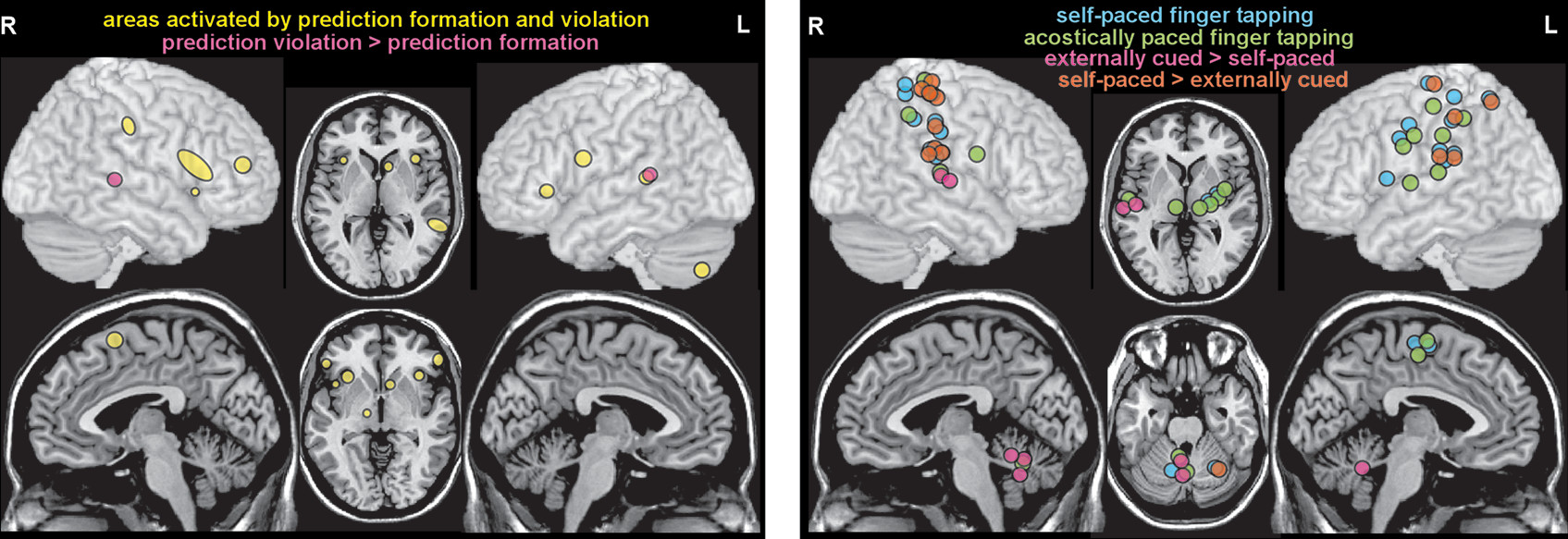
The high dimensionality of temporal processing makes it challenging to combine data across studies in order to identify neural correlates of any single dimension, as multiple other dimensions are likely to be merged in the resulting groupings. Another approach is to focus on how temporal information is used. One meta-analysis combined fMRI and PET studies in three functional domains (music, language, action perception) to test the hypothesis that there is a cohesive network of areas supporting higher level predictive processing (
14). Convergence of activations identified a widespread network of cortical (bilateral: anterior insula, inferior frontal gyrus, ventral premotor cortex; right: presupplementary motor cortex, middle frontal gyrus, supramarginal gyrus; left: posterior superior temporal sulcus/gyrus) and subcortical (right: subthalamus; left: caudate, cerebellum) areas (
Figure 3). Activations associated with prediction formation (i.e., generating an expectation for a future event) and prediction violation (i.e., expected event does not occur) were also compared with test the hypothesis that they are processed by the same network. A contrast analysis between prediction formation and prediction violation identified only a single area (posterior superior temporal sulcus/gyrus) as possibly different, indicating that both primarily engage the same network. Although these results also imply the presence of domain-general (what, when, where) predictive processing, the authors noted that confirmation would require more studies (
14). A recent study tested the hypothesis that subcortical predictive processing differs by source of timing information by comparing patients with Parkinson’s disease (PD) or spinocerebellar ataxia on two subsecond temporal prediction tasks (
47). Performance of the PD group was improved by single interval cues but not by rhythmic cues. In contrast, performance of the spinocerebellar ataxia group was improved by rhythmic cues but not by single interval cues. As the authors discussed, these results are further support for basal ganglia processing of temporal predictions based on rhythm and cerebellar processing of temporal predictions based on intervals (
47).
Another meta-analysis combining fMRI and PET studies compared acoustically paced and self-paced finger tapping to identify the neural basis of sensorimotor synchronization (
15). Movement cued by repetitive sensory stimuli induces motor entrainment to the beat (sensorimotor synchronization) (
15). A comparison to memory-based finger tapping was also included. Convergence of activations for the externally cued and self-paced conditions identified the expected widespread network of both cortical (bilateral: sensorimotor cortex, supplementary motor cortex, inferior parietal cortex; left: ventral and dorsal premotor cortex) and subcortical (bilateral: lateral cerebellum; left: putamen, globus pallidus, thalamus) areas (
Figure 3). Many of these areas were more activated in the self-paced condition. In contrast, the cerebellar vermis was activated in the acoustically paced and memory-based conditions, but not the self-paced condition (
Figure 3). As discussed by the authors, these results support the hypothesis that different subcortical networks are preferentially recruited when external cuing of movement induces sensorimotor entrainment compared with when movements are internally initiated (
15). The therapeutic benefits of incorporating rhythmic auditory stimulation (rhythmic auditory cuing) into motor rehabilitation of patients with PD are well established (
26,
28). The presumed mechanism of action is that induction of sensorimotor synchronization facilitates motor preparation and initiation. The contrast in patients with PD between preservation of one use of rhythm-based timing information and loss of another (i.e., temporal prediction) provides further evidence that neural correlates of temporal processing may differ depending on how the temporal information is used. Although rhythmic auditory stimulation has been primarily incorporated into treatment of PD, it has also been demonstrated to be of benefit in other acquired brain injury conditions (e.g., stroke, traumatic brain injury, multiple sclerosis, cerebral palsy) and developmental disorders (e.g., autism spectrum disorder) (
27,
28).
In summary, although there is still much to be discovered regarding the neural correlates of temporal processing, evidence to date supports some insights. fMRI and PET indicate that multiple cortical and subcortical structures participate. Depending on its intended use, there is parallel and presumably simultaneous processing of timing information. Impairments in specific aspects of temporal processing can be associated with different neuropsychiatric disorders, and successes in rhythm-based interventions support an emerging relevance in clinical management.