Traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) occur at considerably high rates among veterans returning from the conflicts in Iraq and Afghanistan, with about 12%−20% of these veterans reporting an event consistent with mild TBI (mTBI) (
1–
4) and 23% being diagnosed with PTSD (
5,
6). Notably, it may be more common to have both conditions than either disorder alone (
7–
9). Both mTBI and PTSD can result in subjective cognitive, emotional, and physical symptoms and objective cognitive difficulties that overlap and are often difficult to disentangle (
10). The majority of studies evaluating clinical outcomes have found that subjective symptoms and objective neurocognitive deficits resolve within days or weeks following mTBI (
11,
12). However, some studies have identified problematic symptoms and subtle cognitive difficulties months to years postinjury, especially in veteran samples (
8,
13). These persisting symptoms are often related to mental health, psychosocial factors, and litigation status (
14–
16). Moreover, the combination of commonly co-occurring psychiatric disorders (PTSD, depression) with a history of military TBI places veterans at substantial risk for disability in occupational and social functioning (
17).
Within the context of mTBI, certain injury characteristics (subsequently referred to as TBI characteristics), such as mechanism of injury and lifetime number of mTBIs, have been shown to play a role in both the maintenance of postconcussive symptoms and persisting cognitive deficits (
18–
20). Furthermore, symptoms of PTSD—particularly the type and severity of PTSD symptoms—have also been associated with long-lasting postconcussive symptoms and decrements in cognitive performance (
21,
22). Moreover, greater mTBI injury burden in the context of PTSD has been associated with increased suicidal ideation (
23,
24) and poorer functional outcomes (
25) in the veteran population. This, taken together with evidence that cognitive difficulties predict reduced response to PTSD treatment (
26,
27), suggests that a better understanding of independent and synergistic associations between TBI characteristics and PTSD symptom clusters in treatment-seeking veterans has the potential to inform treatment for veterans who served in Iraq and Afghanistan.
The literature regarding the association between TBI characteristics and persistent postconcussive symptoms and cognition across military and nonmilitary samples remains equivocal. In studies examining military samples, a history of multiple TBIs—particularly three or more mTBIs—has been associated with higher reporting of postconcussive symptoms (
19,
28,
29) and reduced neurocognitive functioning and greater intraindividual variability on cognitive measures (
19,
30), even when controlling for PTSD. In contrast, Cooper et al. (
31) did not find differences on self-reported postconcussive symptoms or on a cognitive composite score between service members with mTBI histories and two groups without a history of TBI (history of orthopedic injury or PTSD). However, they identified a nonsignificant pattern of stepwise increases in subjective distress with increasing number of mTBIs.
In terms of mechanism of injury, the majority of studies evaluating military service members have compared those with a history of blast-related mTBI to those with mTBI from other mechanisms (e.g., blunt force) and have found largely similar postconcussive symptom reporting and neuropsychological performance across groups (
18,
32–
36). However, there is some evidence to suggest that a history of blast exposure—regardless of whether or not the exposure resulted in an mTBI—is associated with higher self-reported postconcussive symptoms (
18,
37) and reduced executive functioning, processing speed, and verbal memory performance (
38–
40), although not all studies included PTSD as a covariate.
In addition to TBI characteristics, severity of PTSD symptoms has also been strongly associated with both self-reported postconcussive symptoms (
35,
40) and objective neurocognitive functioning (
41). In fact, PTSD along with other psychological and behavioral conditions has been demonstrated to have strong associations with neurobehavioral symptoms and cognitive dysfunction, whereas mTBI is only weakly related, particularly when taking these other factors into account (
42,
43). More nuanced approaches have found that memory (
44) as well as aspects of executive functioning, including response inhibition, suppression of distractors, and capture of attention may be particularly vulnerable to PTSD (
45,
46). Although several studies have evaluated the influence of comorbid PTSD and a history of mTBI on cognition and postconcussive symptoms relative to either condition alone with mixed findings (
47–
50), the individual contributions of specific PTSD symptom clusters have been less frequently evaluated in veterans with PTSD and a history of mTBI. A better understanding of how specific symptom clusters of PTSD relate to outcomes may be particularly informative in monitoring and treating veterans with subthreshold PTSD, primarily those who endorse clinically meaningful symptoms in certain, but not necessarily all, cluster domains. In fact, some previous work suggests that poor outcomes, including neurocognitive dysfunction in certain domains, may depend on the particular profile of symptoms observed (
21,
51).
In one study of 40 Iraq and Afghanistan veterans with PTSD (N=30 with a history of mTBI), Swick et al. (
52) identified a positive association between impaired response inhibition and all PTSD symptom clusters on the PTSD Symptom Checklist (PCL) using the DSM-IV three-factor model (re-experiencing, avoidance/emotional numbing, and hyperarousal), with the strongest relationship being with re-experiencing symptoms. However, they did not include measures of other cognitive domains or a measure of performance validity, factors that are important to address in this population (
53,
54). Another study evaluating the three-factor model of the PCL in veterans found significant associations between the hyperarousal cluster and postconcussive symptoms when removing postconcussive symptoms that overlap with PTSD or depression, but they did not include measures of objective cognitive functioning (
22).
A four-factor model of PTSD symptoms that splits avoidance and numbing symptoms into two separate factors was found to improve fit over the original DSM-IV three-factor model (
55), including in veterans with mTBI or close-range blast exposure history (
56). In the only known study to evaluate the four-factor model of PTSD in relation to objective cognition among Iraq and Afghanistan veterans with and without PTSD, poorer visual memory performance was associated with higher PTSD symptoms in all clusters, and poorer verbal learning was associated with higher avoidance and numbing symptoms (
21). Aase et al. (
21) used only a single measure to assess cognition in each domain, however, and did not include a measure of self-reported postconcussive symptoms.
Although there is preliminary support for the proposal that TBI characteristics and greater severity of PTSD symptoms in certain clusters may confer additional risk for poor outcome following mTBI, no studies to date have examined possible synergistic effects on self-reported postconcussive symptoms and objective measures of cognitive performance. Additionally, there is a large body of literature suggesting that symptom overreporting and performance below expectations on cognitive validity measures are significant issues in this population (
53,
54). This is particularly an issue within clinical settings (
57), but can occur even in research contexts (
58). However, not all studies referenced above have adequately accounted for these factors. The present study sought to evaluate the associations between TBI characteristics and PTSD symptom clusters on postconcussive symptoms and cognition (memory, processing speed, and executive functioning), as well as the interaction between TBI characteristics and PTSD symptom clusters, in treatment-seeking Iraq and Afghanistan veterans. Veterans with performance below expectations on performance and symptom validity measures were excluded from the study to reduce the influence of overreporting of clinical symptoms and invalid cognitive data.
First, we hypothesized that repetitive and blast-related mTBI would be associated with poorer cognitive performance and higher self-reported postconcussive symptoms (
19,
29,
38–
41). Second, given the nascent research into PTSD symptom clusters and the varying models of PTSD factor structure across studies, the analyses regarding relationships between specific PTSD symptom clusters and cognition/postconcussive symptoms were exploratory in nature and no specific hypotheses were generated. Third, we hypothesized that TBI characteristics would moderate the relationship between PTSD symptom clusters and cognition/postconcussive symptoms such that in those with repetitive and blast-related mTBI, higher PTSD symptoms across clusters would be associated with poorer cognition and higher postconcussive symptoms.
Methods
Participants and Procedure
Participants were Iraq and Afghanistan veterans with a history of mTBI, a current PTSD diagnosis, and self-reported cognitive complaints who were given a comprehensive neuropsychological assessment at baseline as part of a treatment study described previously (
53,
59). For the purpose of the present study, mTBI was defined as self-reported loss of consciousness ≤30 minutes, alteration of consciousness up to 24 hours, and/or posttraumatic amnesia less than or equal to 24 hours, according to the VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury (
60,
61). Current PTSD diagnosis based on DSM-IV criteria was confirmed by either a chart review of a diagnostic clinical interview conducted by a mental health professional or the administration of a structured diagnostic clinical interview (the Clinician-Administered PTSD Scale for DSM-IV), as has been done in previous studies (
27,
59,
62).
A total of 100 participants were enrolled in the parent study and attended the baseline neuropsychological assessment appointment. The following exclusion criteria for the present study were assessed using medical chart review of diagnostic clinical interviews conducted by mental health professionals and detailed screening questions of the presence of psychiatric disorders: active alcohol or substance dependence defined by DSM-IV criteria (
63); suicidal intent or attempt within the previous month; current psychotic disorder; Wide Range Achievement Test–Fourth Edition (
64) (WRAT-4); Reading subtest standard score <75 (due to the possibility of a learning disorder); performance below standard cutoffs on performance validity measures; a score ≥33 on the Validity-10 (
65), an embedded symptom validity measure within the Neurobehavioral Symptom Inventory (
66) (NSI); a history of moderate or severe TBI; and missing more than one cognitive measure from a cognitive composite domain. For the present study, 33 veterans were excluded from the sample of 100 due to WRAT-4 Reading standard score below 75 (N=2), missing more than one cognitive measure from a cognitive composite domain (N=2), history of moderate TBI (N=6), and performance below expected cutoffs on any of the three performance validity measures described below or above a score of 33 on the embedded symptom validity measure described above (N=25). A total of 67 veterans were included in the present study (two veterans fell into two of these exclusion categories).
All procedures were approved by the Institutional Review Board of the Veterans Affairs San Diego Healthcare System (VASDHS), and all participants provided written informed consent. Participants were veterans enrolled in Veterans Affairs services and subsequently enrolled in the parent treatment study (i.e., treatment-seeking veterans) and were recruited through advertisements and informational sessions at various clinics within the VASDHS, as well as veteran centers at local colleges.
TBI Diagnosis and Postconcussive Symptoms
The Warrior Administered Retrospective Casualty Assessment Tool (
67) is a structured interview that was used to collect standardized information regarding participants’ TBI history. This measure gathers information about the number of TBIs, presence/duration of loss of consciousness and posttraumatic amnesia, and the mechanism of injury for each TBI. Consistent with the methodology of several previous publications, the number of lifetime TBIs was dichotomized into one to two prior mTBIs versus three or more (
19,
28–
30). Postconcussive symptoms were assessed using the NSI, a 22-item self-report measure that assesses symptoms occurring over the past 2 weeks on a Likert-type scale.
PTSD Symptomatology
PTSD symptomatology was assessed using the PTSD Checklist–Specific Trauma (
68) (PCL-S). The PCL-S is a self-report measure that prompts respondents to rate how much they were bothered by all 17 DSM-IV items of PTSD (
63) in the past month. Items are rated on a Likert-type scale ranging from 1 (not at all) to 5 (extremely). A total score can be obtained by adding all 17 items (range, 17–85), and it has been demonstrated to have a four-factor structure: re-experiencing, avoidance, hyperarousal, and numbing symptoms (
56,
69,
70).
Neuropsychological Tests
Performance validity was assessed using the Test of Memory Malingering Trial 2 and Retention Trial (
71) and the California Verbal Learning Test–Second Edition (CVLT-II) Forced Choice trial (
72). Failure (as determined by standard published cutoffs in the manuals) on even one of these tests warrants consideration of poor performance validity among Iraq and Afghanistan veterans with a history of mTBI (
73).
Because our hypotheses were specifically related to the neuropsychological domains of verbal memory, processing speed, and executive functioning, the following measures were selected from a larger battery for their sensitivity to impairments commonly observed in those with a history of mTBI and PTSD (
74,
75). CVLT-II List A 1–5 Total Recall, Short Delay Free Recall, and Long Delay Free Recall measures were used for memory (
72). In the domain of processing speed, the Symbol Search and Coding subtests were used from the Wechsler Adult Intelligence Scale–Fourth Edition (
76) (WAIS-IV); subtests from the Color Word Interference Test (Color Naming and Word Reading) and Trail-Making Test (Visual Scanning, Number Sequencing, Letter Sequencing, and Motor Speed) were used from the Delis-Kaplan Executive Function System (D-KEFS) (
77). With regard to executive functioning, subtests were used from the D-KEFS Color Word Interference Test (Inhibition and Inhibition Switching) and D-KEFS Trail-Making Test (Number-Letter Switching), as well as perseverative responses and errors, nonperseverative errors, and total errors from the Wisconsin Card Sorting Test–64 Card Version (WCST-64) (
78). Finally, the WRAT-4 Reading subtest standard score was used as a measure of premorbid intellectual functioning.
In order to reduce the likelihood of type I errors and limit the number of analyses, cognitive composite scores were created. For the measures in which higher raw scores indicate worse performance (all Color Word, Trail-Making, and WCST scores), the raw scores were inverted and then converted into standardized z scores. For the measures in which higher raw scores indicated better performance, raw scores were converted directly into standardized z scores. Scores from each domain were then averaged to create three composite scores with higher z scores indicating better performance. Cronbach’s alpha was 0.90 for the memory composite, 0.89 for the processing speed composite, and 0.79 for the executive functioning composite, indicating high internal consistency for each composite. The intercorrelations between the composites ranged from r=0.24 to r=0.41.
Data Analysis
Because raw data for cognitive variables were used in all analyses, associations between cognitive composites and demographic variables (age, education, gender, WRAT reading score) were assessed, and any variables significantly related to cognitive performance were included as covariates in all analyses with that outcome variable. To examine associations between TBI characteristics and cognitive performance/self-reported postconcussive symptoms (aim 1), independent samples t tests were conducted with each of the three cognitive composite scores and the NSI total score as dependent variables and mechanism of injury (blast versus nonblast) or lifetime number of mTBIs (≥3 versus <3) as independent variables. To examine relationships between PTSD symptoms and cognitive performance/postconcussive symptoms (aim 2), Pearson correlations were used to identify significant associations between study variables. For any significant associations between the PCL-S total score and cognitive composites or the NSI, all four PTSD symptom clusters (re-experiencing, avoidance, hyperarousal, and numbing) were then entered as independent variables into a regression analysis predicting the cognitive composite score and/or NSI. Finally, to assess interactive relationships (aim 3), interaction terms were created using TBI characteristics and PTSD symptom clusters found to be associated with objective cognition or postconcussive symptoms to determine whether TBI characteristics moderated relationships between PTSD symptom clusters and cognition/postconcussive symptoms. It was determined a priori that although applying a more conservative p value would reduce the probability of type I error, these corrections were considered too stringent, given the relatively small sample size. As a result, we chose to use a more liberal criterion of p<0.05 to determine significance, in congruence with recommendations by Rothman (
79). In short, Rothman argued that controlling for multiple comparisons can lead to errors in interpretation when using actual data versus random numbers and prevent findings from being published that warrant future replication and exploration. Effect sizes are provided to aid in interpretation.
Results
On average, veterans in the present sample had a mean age of 34.76 years (SD=8.15), with 13.79 (SD=1.98) years of education. Regarding deployment history, 28% were deployed once, 36% were deployed twice, 15% were deployed three times, and 15% were deployed four or more times. Approximately half of the sample (51%) had three or more lifetime mTBIs; the majority of veterans (63%) experienced at least one blast-related mTBI in their lifetime. Of those with three or more lifetime mTBIs, 22 had a history of blast-related mTBI and 12 did not. All veterans were in the postacute phase following their most recent mTBI (mean years postinjury=5.22 [SD=3.65]). On average, veterans reported a moderate level of depressive symptoms and difficulties involving sleep and pain. Complete descriptive statistics of the demographic, injury, and mental health variables are presented in
Table 1.
Prior to the main analyses, preliminary analyses tested for significant demographic (gender, age, education, WRAT reading standard score) predictors of outcome variables (
Table 2). Age was negatively associated with the executive function composite (r=−0.33, df=65, p=0.006). Thus, age was included as a covariate in all analyses in which the executive function composite was the dependent variable. No other significant associations were observed between demographic variables and cognitive composites or postconcussive symptoms (all p values >0.05).
Association Between TBI Characteristics and Cognitive Performance/Postconcussive Symptoms
With regard to cognition, veterans with a history of blast-related mTBI performed worse on the processing speed composite (t=2.95, df=65, p=0.004), but not on the memory (t=−0.96, df=65, p=0.340) or executive functioning composites (F=0.70, df=1, 64, p=0.407) compared with those without a history of blast-related TBI. As for the relationship between number of mTBIs and cognition, the cognitive composites—memory, processing speed, and executive functioning—did not differ between those with one to two versus three or more lifetime mTBIs (all p values >0.05).
With respect to postconcussive symptoms, veterans with or without a history of blast-related mTBI did not differ on the NSI total score. Similarly, veterans with one to two versus three or more lifetime mTBIs did not differ on the NSI total score (all p values >0.05).
Association Between PTSD Symptom Clusters and Cognitive Performance/Postconcussive Symptoms
As shown in
Table 2, the executive functioning composite was the only cognitive composite significantly associated with the PCL-S total score, such that higher PTSD symptoms were associated with poorer executive functioning performance when controlling for age. Thus, the four PTSD symptom clusters were entered into linear regressions with the executive functioning composite as the dependent variable, controlling for age. Full statistics for the regression analyses are shown in
Table 3. Hyperarousal was the only PTSD symptom cluster that was significantly related to executive functioning (β=−0.37, p=0.025; all other p values >0.05).
Postconcussive symptoms (as measured by the NSI total score) were significantly and positively correlated with the PCL-S total score (
Table 2). When the four PTSD clusters were entered into a regression model with the NSI total score as the dependent variable, only the hyperarousal cluster was significantly and positively associated with higher postconcussive symptoms (β=0.46, p=0.005) (
Table 3).
Interactions Between TBI Characteristics and PTSD Symptom Clusters on Cognitive Composites and Postconcussive Symptoms
Interaction between blast history and PTSD symptom clusters on cognitive composites.
Because blast history was associated with poorer processing speed performance, four interaction terms were created (blast history-by-re-experiencing, blast history-by-avoidance, blast history-by-hyperarousal, and blast history-by-numbing) to determine whether relationships between PTSD symptom clusters and cognition were moderated by blast-related mTBI. Each of these interaction terms was entered into separate regressions with the corresponding PTSD cluster score and the dichotomous blast-related mTBI variable. As shown in
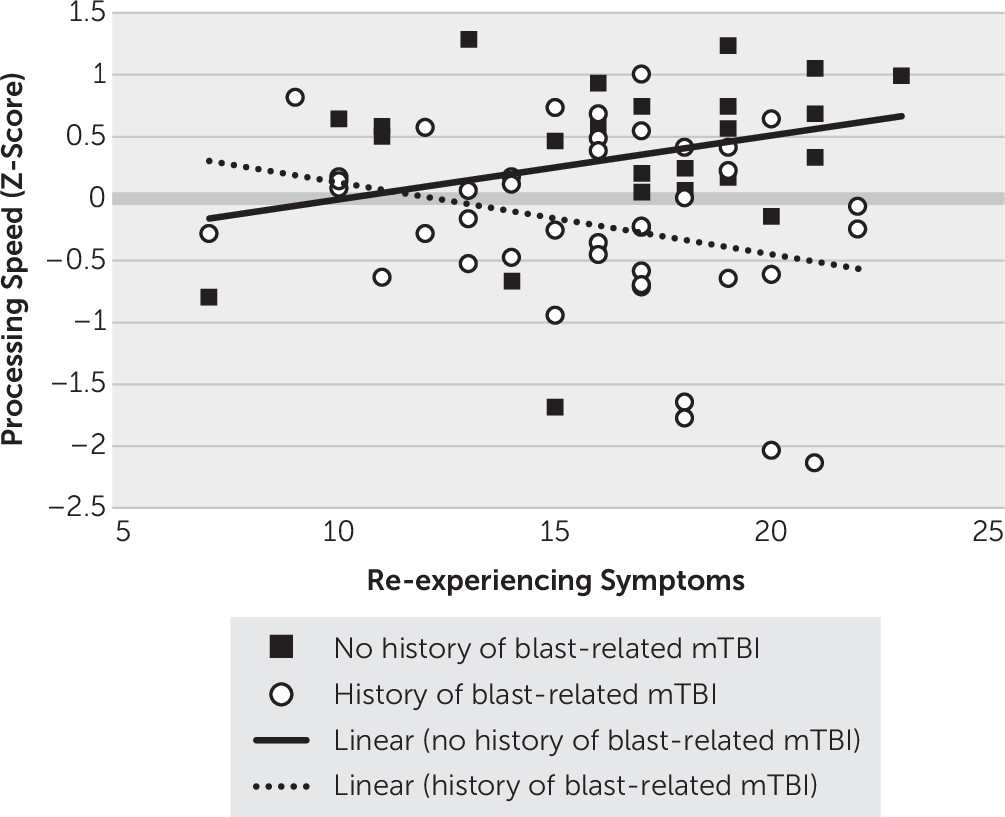
Figure 1, the only significant interaction that emerged was between a history of blast-related mTBI and re-experiencing symptoms (b=−1.182, p=0.025): veterans with a history of blast-related mTBI demonstrated a pattern of worse processing speed associated with higher re-experiencing symptoms (b=−0.058, 95% CI=−0.119, 0.003, t=−1.91, p=0.060), whereas veterans without a history of blast-related mTBI did not demonstrate a significant association between processing speed and re-experiencing symptoms (b=0.052, 95% CI=−0.022, 0.125, t=1.41, p=0.164).
Interactions between hyperarousal and TBI characteristics on cognitive composites.
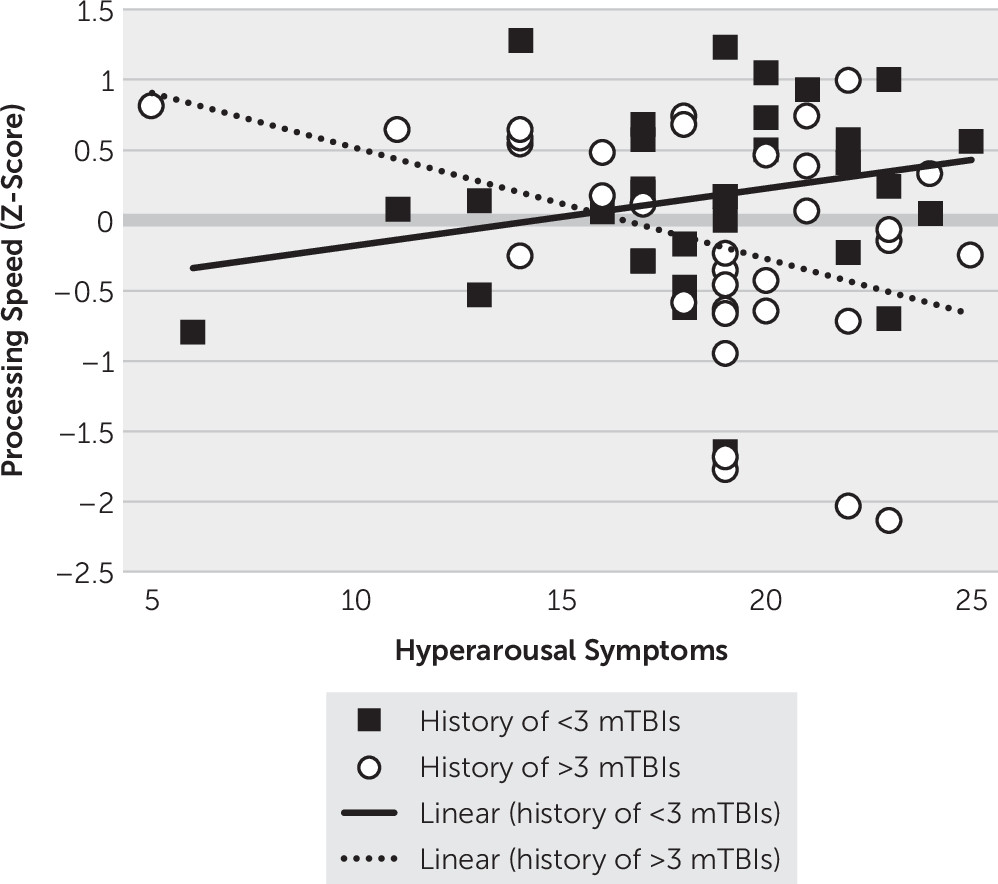
Because hyperarousal was associated with the executive functioning cognitive composite, two interaction terms were created with hyperarousal and the dichotomous TBI characteristics (hyperarousal-by-blast history and hyperarousal-by-number of lifetime mTBIs) to determine whether TBI characteristics moderated the association between hyperarousal symptoms and cognitive performance. Each interaction term was then entered into regression models separately, with the interaction term, hyperarousal cluster scores, and the corresponding TBI characteristic (history of blast-related mTBI, lifetime number of mTBIs) in each model. The only significant interaction that emerged was between lifetime number of mTBIs and hyperarousal symptoms with regard to the processing speed composite (b=−1.54, p=0.009). As shown in
Figure 2, veterans with a history of three or more lifetime mTBIs demonstrated worse processing speed associated with higher hyperarousal symptoms (b=−0.079, 95% CI=−0.140, −0.017, t=−2.55, p=0.013), whereas veterans with a history of fewer than three TBIs did not demonstrate a significant association between processing speed and hyperarousal symptoms (b=0.040, 95% CI=−0.023, 0.103, t=1.28, p=0.204).
Interactions between hyperarousal and TBI characteristics on postconcussive symptoms.
Because hyperarousal was associated with postconcussive symptoms, the two interaction terms (hyperarousal-by-blast history and hyperarousal-by-number of lifetime mTBIs) were entered into regression models separately, with the interaction term, hyperarousal cluster scores, and the corresponding TBI characteristic (history of blast-related mTBI, lifetime number of mTBIs) in each model to determine whether TBI characteristics moderated the association between hyperarousal symptoms and postconcussive symptoms. There were no significant interactions between TBI characteristics and hyperarousal symptoms with regard to postconcussive symptoms (all p values >0.05).
Discussion
The objective of the present study was to evaluate independent and synergistic associations between PTSD symptom clusters and TBI characteristics on self-reported postconcussive symptoms and objective cognition among treatment-seeking Iraq and Afghanistan veterans with PTSD and a history of mTBI. Higher PTSD symptoms, particularly hyperarousal, were associated with poorer executive functioning and higher postconcussive symptoms. Furthermore, TBI characteristics (mechanism of injury and lifetime number of mTBIs) moderated relationships between processing speed and PTSD symptom clusters of re-experiencing and hyperarousal, such that for those with blast-related or repetitive mTBI, greater re-experiencing and hyperarousal symptoms, respectively, were associated with reduced performance on objective tests of processing speed.
The observed associations between PTSD symptoms and objective cognition are consistent with a large body of literature in civilians and veterans suggesting enduring cognitive compromise associated with psychiatric symptoms in those with a history of mTBI (
80–
82), particularly on tasks of switching and attention/working memory among those with PTSD (
41,
48,
51,
83). With regard to specific PTSD symptom clusters, direct associations were identified between hyperarousal and both executive functioning and self-reported postconcussive symptoms. Additionally, for those with greater injury burden (blast and repetitive mTBI), re-experiencing and hyperarousal symptoms were associated with reduced speed of processing. These results were somewhat consistent with the findings of Swick et al. (
52), in which PTSD symptom clusters were associated with executive functioning, although they found that executive dysfunction was most strongly associated with re-experiencing symptoms rather than hyperarousal. The present findings were contrary to those of Aase et al. (
21), in which worse verbal learning was associated with greater avoidance and numbing. Various methodological factors (e.g., use of a single measure within a cognitive domain, lack of performance validity measures, differing models of PTSD factors, inclusion of those without a history of mTBI, without PTSD, or subthreshold PTSD) may account for the discrepant findings.
The mechanisms underlying these relationships (hyperarousal symptoms and executive functioning and processing speed, re-experiencing symptoms and processing speed) may be related to difficulty efficiently shifting attention in PTSD (
84–
86). With regard to hyperarousal, difficulty controlling and shifting attention away from trauma-related associations could hinder the ability to contextualize stimuli, thus leading to exaggerated responses even in safe contexts (
87). Difficulties with these functions, as well as the speed with which these processes can take place, may affect the ability to effectively manage emotional and physiological reactions to triggers even loosely related to the trauma memory (
88). It is likely that the relationship between cognition and psychological distress is bidirectional (
89), in which symptoms of PTSD such as hyperarousal also hinder the ability to perform well on both neuropsychological tests and cognitive tasks required in everyday life.
The results of our study do not support any independent associations between TBI characteristics and objective cognitive functioning. However, TBI characteristics (history of blast or repetitive mTBI) conferred additional risk for poorer processing speed in the presence of higher re-experiencing and hyperarousal symptoms. This finding is somewhat consistent with a retrospective record review study demonstrating slower reaction time and higher PTSD symptoms in Army service members with multiple blast mTBIs (
90). The mechanisms underlying the interactions between TBI characteristics and PTSD symptom clusters on processing speed in the present study are not entirely clear; however, it is possible that neurocognitive inefficiencies that are present acutely after mTBI may affect the development and course of PTSD (
36,
91,
92) and/or that neurobiological alterations associated with PTSD may cause any neurocognitive inefficiencies to persist (
93,
94). Furthermore, associations between re-experiencing symptoms and weakened resting-state connectivity in frontolimbic systems (
95), as well as reductions in white matter integrity underlying these connections (
96,
97), suggest that a history of mTBI may disrupt the network responsible for protecting trauma associations from entering working memory.
Porter et al. (
22) also found postconcussive symptoms were positively related to hyperarousal but not history of mTBI. This association was present even when postconcussive symptoms overlapping with PTSD and depression were removed. Prior research on the convergence between objective and subjective cognitive performance has been equivocal (
98). However, in the present study, those with greater hyperarousal demonstrated worse objective cognitive performance and subjective complaints, suggesting that veterans with executive dysfunction may experience more interruption in their daily functioning. Furthermore, they may be more aware of and likely to report these difficulties, which may in turn produce even more distress and associated physiological reactivity. Thus, veterans reporting high postconcussive symptoms as well as high hyperarousal symptoms may also be experiencing objective executive functioning difficulties and therefore may benefit from comprehensive neuropsychological testing or augmented interventions to compensate for their cognitive difficulties.
Although this study has notable strengths, there are also several limitations that must be acknowledged. First, the sample was small, and analyses should be replicated in larger samples. Next, data were cross-sectional and thus it is unknown whether deficits in cognition predate and/or exacerbate PTSD symptoms or vice versa. We also did not have a group with PTSD but without a history of mTBI to determine whether mTBI history exerts effects on PTSD symptom clusters over and above what is observed in PTSD alone. Future research would benefit from inclusion of a relevant, treatment-seeking, PTSD-only group for comparison. However, given diversity within the mTBI sample (mechanism and number of injuries), as well as the treatment-seeking nature of the sample, the present study has implications for treatment (discussed further below). Commonly co-occurring conditions such as depression, pain, and sleep were not assessed using a diagnostic interview and are important variables to include in future research on this population. Another limitation was that a self-report measure was primarily used to assess PTSD symptoms; however, self-report measures have been shown to have high correspondence with clinician-administered measures (
99,
100), and this approach has been used previously (
27,
59). Finally, the DSM-IV PTSD criteria were used in the present study rather than the updated DSM-5 criteria. Given that many longitudinal studies will likely continue to collect DSM-IV PTSD data, and that the PTSD DSM-IV symptom clusters are still present in DSM-5 (
101), the present study provides relevant information on how these symptom clusters relate to important variables in those with PTSD and a history of mTBI.
The specific relationships between re-experiencing and hyperarousal symptoms of PTSD and cognition and self-reported postconcussive symptoms have implications for treatment of those with PTSD and a history of mTBI, especially given that pretreatment characteristics such as cognitive performance, PTSD symptoms, and mechanism of injury have been shown to predict treatment retention and response in veterans with PTSD and/or a history of mTBI (
26,
27,
102). The findings from the present study indicate that those with greater mTBI injury burden exhibit associations between slowed processing and higher re-experiencing and hyperarousal symptoms and suggest that treatment should more directly target these symptoms. For example, cognitive rehabilitation before or concurrently with PTSD treatment holds promise to boost treatment compliance and gains in those with comorbid PTSD and mTBI history (
27,
59). It may also be of benefit to clinical providers to understand that these cognitive difficulties may play a role in the maintenance of re-experiencing and hyperarousal symptoms or vice versa, particularly if they are refractory to treatment. This may be especially relevant for veterans presenting with subtle processing speed or executive functioning difficulties with a history of blast or repetitive mTBI. Finally, a recent study demonstrated that PTSD moderated the interaction between a history of a single mTBI and executive dysfunction in late middle-aged Vietnam-era male veterans, suggesting that the synergistic association between these variables may continue to be important as Iraq and Afghanistan veterans age (
103). Taken together, our findings suggest that ongoing evaluation of the independent and synergistic influences of TBI characteristics and PTSD symptom clusters on cognition and postconcussive symptoms among veterans across the lifespan is warranted.