Servicemembers commonly report fatigue for months after returning from deployment. In a prospective study of Dutch Armed Forces deployed to Afghanistan, 14% of returning troops endorsed recurrent, severe fatigue for the 24 months of the study duration (
1). Mild traumatic brain injury (mTBI) in military and nonmilitary populations is also frequently associated with persistent fatigue (
2,
3). Fatigue is an important clinical finding that has been shown to affect duty performance. For example, daytime fatigue was found to be a significant contributor to poor memory performance in Iraq and Afghanistan veterans, independent of mTBI, posttraumatic stress disorder (PTSD), or sleep quality (
2). The neural basis of fatigue is poorly understood, particularly in the setting of neuropsychiatric disease, which inhibits the development of effective treatments.
Most studies of the neural correlates of pathological fatigue have been observed in patients with single diagnoses and localizable pathology, such as studies of multiple sclerosis (
4) and some, but not all, studies of poststroke fatigue (
5). One hypothesis arising from this work is that fatigue may represent disturbed reward processing from disturbance of striatal-prefrontal cortical connections, resulting in an effort-for-reward mismatch (
6).
Striatal-prefrontal cortical connections are believed to be involved in balancing the energetic cost of action against potential reward. Higher energetic cost relative to predicted reward may decrease the willingness to act, resulting in a state that may correspond with the subjective experience of fatigue. The proposed cortical and subcortical structures involved in effort/reward action control include the nucleus accumbens in reward anticipation, the putamen in the calculation of energetic cost of an action, the anterior cingulate in effort/reward valuation, and the orbitofrontal cortex in regulation of vigorous output toward a goal (
7,
8). Positive correlations between self-reported fatigue and mean diffusivity among basal ganglia nuclei have been reported in healthy young adults (
9). Increased striatal-prefrontal functional connectivity has also been observed in some patients with depression as well and has been found to correlate with increased anhedonia and psychomotor retardation (
10).
The primary aim of this exploratory study was to investigate the neural correlates of fatigue dimensions in U.S. servicemembers and veterans experiencing at least one deployment-related concussion occurring during deployment in Iraq and Afghanistan in support of Operation Enduring Freedom (2001–2014), Operation Iraqi Freedom (2003–2010), and Operation New Dawn (2010–2011). The study was developed to test the hypothesis that if fatigue involves disrupted effort/reward processing, this should manifest as altered basal ganglia functional connectivity, as observed in other amotivational states. We used a multidimensional self-report of fatigue and resting-state functional connectivity measured with functional MRI (fMRI). We also acquired task-related functional connectivity data, which will be reported elsewhere.
Methods
Participants
Twenty-eight active and former servicemembers (100% male) with a history of military deployment and persistent symptoms at least 6 months following an mTBI during a deployment were recruited. Persistent symptoms included fatigability, sleep disturbances, headache or other chronic widespread pain, emotional lability, lack of spontaneity or apathy, loss of motivation, irritability, and change in personality or anxiety. Each interested individual was initially interviewed by the study coordinator by phone using a checklist that listed the Department of Defense (DoD) definition of mTBI (
11) used in this study, study inclusion and exclusion criteria, and the persistent symptoms listed above. The lead investigator then interviewed each participant in person to obtain an injury history. The DoD definition of mTBI was used (
11). Demographic and clinical characteristics of the study participants are presented in
Table 1. Individuals with daily use of stimulants (including >600 mg caffeine), narcotics, hypnotics, or anxiolytics were excluded, as were those with sleep apnea. Prior to participation, all participants gave informed consent to a protocol approved by the National Institutes of Health Combined Neuroscience Institutional Review Board. All study procedures conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki) and all U.S. Department of Health and Human Services regulatory standards.
Outcome Measures
The primary outcome measures for this study were scores from the Multidimensional Fatigue Inventory (MFI) (
12). The MFI produces a score for each of five subscales: mental fatigue, physical fatigue, general fatigue, reduced motivation, and reduced activity. To control for the confounding effects of depression on self-reported fatigue, participants also completed the Center for Epidemiologic Studies Depression (CESD) Scale (
13). Participants also completed the Posttraumatic Stress Disorder Checklist—Military Version (
14).
Participants completed a 7.5-minute resting-state fMRI scan and a T1-weighted magnetization-prepared rapid acquisition gradient echo (anatomical) scan during a single session in a Siemens 3T Biograph scanner. The resting-state scan included 150 T2*-weighted echoplanar volumes (time to repeat=3,000 ms, echo time=25 ms, flip angle=90°, slice thickness=3 mm, slices=43, voxel size=1.7188 × 1.7188 × 3 mm, field of view=1,540 × 1,540 mm, matrix=128 × 128). Participants were asked to keep their eyes closed but not to sleep. No music was used during the scan. One participant did not complete the resting-state scanning session due to claustrophobia; another participant’s resting-state data set was unusable due to excessive motion/scanner artifact, resulting in a total of 26 participants for MRI analysis. The MRI acquisition was completed within 24 hours of outcome measures assessments.
Preprocessing of the resting-state fMRI data included despiking, interpolation to correct for slice time acquisition differences (3dTshift), and spatial registration to minimize effects of head motion (3dvolreg to the first volume) using Analysis of Functional NeuroImages (AFNI) (
15). The resting-state time-series was blurred using a full width at half maximum of 6 mm, and rescaled to percentage signal change. Anatomical images were segmented into maps of gray matter, white matter, and CSF using FreeSurfer (
16). Then, time-series within the resulting white matter and CSF masks were averaged. Next, we used the ANATICOR procedure to remove artifacts, including six motion parameters, localized average white matter (within a 15-mm-radius sphere centered on each voxel) and CSF time-series, and respiration and cardiac signals (
17,
18). These artifacts were entered into a linear regression with the resting-state time-series (
19). The residual time-series data were then transformed into Talairach space (
20) and standardized to z scores using Fisher’s transformation. Global gray matter was not regressed out to avoid the likelihood of increased anticorrelations (
21,
22).
Functional Connectivity Analyses
Whole-brain correlations were performed for all five MFI subscale scores, controlled for depression using the CESD scale score as a covariate, with a voxelwise threshold of p<0.005, familywise error corrected at p<0.00083. We chose to adjust for the CESD score because some subtypes of depression show changes in striatal-prefrontal connectivity (
10). Whole-brain correlations, also referred to as “connectedness,” are calculated as the average Pearson’s correlation of each voxel with the rest of the brain. Whole-brain connectedness maps were calculated for each participant, and each of the five MFI scales were then correlated in a voxelwise manner across participants.
Seed regions for six basal ganglia nuclei (left and right caudate, left and right putamen, and left and right globus pallidus) were created for each participant using FreeSurfer. Functional connectivity correlations with these six seed regions were performed using the MFI subscale scores (mental fatigue, physical fatigue, general fatigue, reduced motivation, reduced activity). To control for depressive symptoms, the CESD scale score was added as a covariate in the seed-based analyses as well.
Specifically, we performed group-level t tests (one-sample 3dttest++ against zero) of each subject’s scores with the connectivity data, including only areas where 90% of the subjects had data. To control for different motion levels across subjects, a summary measure of each subject’s transient head motion during the scan (AFNI’s @1dDiffMag, comparable to mean framewise displacement) was calculated and entered as a mean-centered covariate into the group-level t tests. We used a voxelwise threshold of p<0.005, corrected to p<0.00083 (familywise error corrected) with a cluster size threshold of K=363, obtained using AFNI’s empirically modeled spatial auto-correlation function (
23).
Results
We regressed whole-brain resting-state functional connectivity against each of the five MFI subscale scores, using a voxelwise threshold of p<0.005 and familywise error-corrected p value of 0.00083, and found no significant correlations.
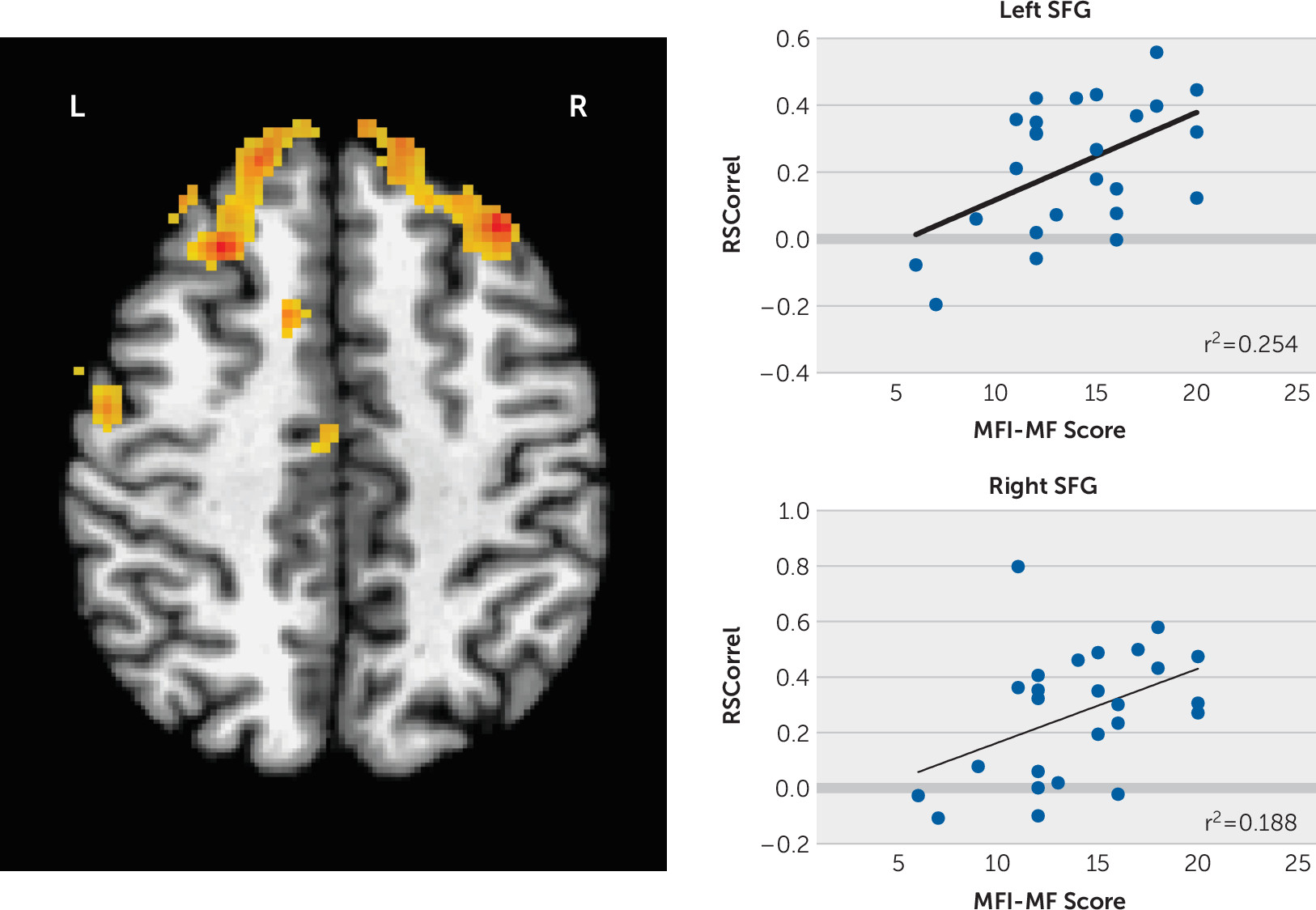
We then regressed the seed region to whole-brain functional connectivity against the MFI subscale scores using a corrected p<0.00083 (familywise error corrected) and found significant positive correlations between the left putamen seed and superior frontal gyri (SFG) bilaterally with mental fatigue adjusted for the CESD score (left SFG MNI coordinates −33, +31, +42, 688 voxels; right SFG MNI coordinates +25, +27, +44, 570 voxels;
Figure 1). No correlations with the other MFI subscale scores survived multiple comparisons correction.
Discussion
The neural basis for the experience of fatigue is unclear. In the effort/reward model of fatigue, effort is overvalued relative to the anticipated reward. Because the putamen is involved in the energetic cost of action, our finding of increased connectivity between the putamen and SFG and mental fatigue ratings is consistent with this model. Our findings also support the hypothesis that fatigue involves altered basal ganglia functional connectivity.
Changes in striatal-prefrontal networks have been reported previously in fatigue. For example, increased connectivity between the left caudate nucleus and left superior frontal gyrus after a fatiguing mental task has been reported in multiple sclerosis patients with cognitive fatigue (
24); in mTBI, a significant correlation between self-reported fatigue and functional connectivity between the middle frontal gyrus and the rest of the brain was found during a psychomotor vigilance task (
25).
Besides history of military deployment and concussion, another consideration in servicemembers and veterans is the possible influence of PTSD on fatigue. One study has looked at connectivity changes in military servicemembers with a history of mTBI with and without PTSD symptoms and found increased connectivity in the posterior left middle frontal gyrus, left parahippocampal gyrus, and left cuneus in those endorsing PTSD symptoms (
26). The reported regions were posterior to the regions we found in our seed-based analysis of fatigue and regions not associated with striatal-prefrontal networks like depression. Given our relatively small sample size and concern for introducing type 2 error, we did not include PTSD as an additional covariate in our analysis.
There are several limitations to this study, one of which is that the correlations are based upon self-report measures, unconfirmed by behavioral or clinical outcomes. However, we were specifically interested in the subjective sense of fatigue, which is available only by self-report and has been shown to be clinically meaningful (
27). Another important limitation is that the individuals in this study were deployed servicemembers and veterans who received at least one in-theater concussion. Because fatigue may be related specifically to deployment independent of concussion history, we cannot causally relate fatigue to either deployment alone or history of mTBI in this exploratory study. Finally, MFI scores have not been reported in other studies of military personnel, so comparisons between military groups with and without a history of mTBI are not currently possible.
Fatigue is a frequent complaint among servicemembers and veterans returning from deployment, including those with a history of mTBI. This study provides preliminary evidence that self-reported mental fatigue correlates with increased striatal-prefrontal functional connectivity and may represent a bias in effort/reward processing. Further work to compare fatigue symptoms in other populations, such as deployed personnel without head injury, is needed to determine if postdeployment fatigue in general is due to such biased processing. Successful treatment of persistent fatigue in servicemembers following deployment will significantly improve their productivity, sense of self-efficacy, and quality of life.