The Diagnostic Challenge of Young-Onset Dementia Syndromes and Primary Psychiatric Diseases: Results From a Retrospective 20-Year Cross-Sectional Study
Abstract
Objective:
Methods:
Results:
Conclusions:
METHODS
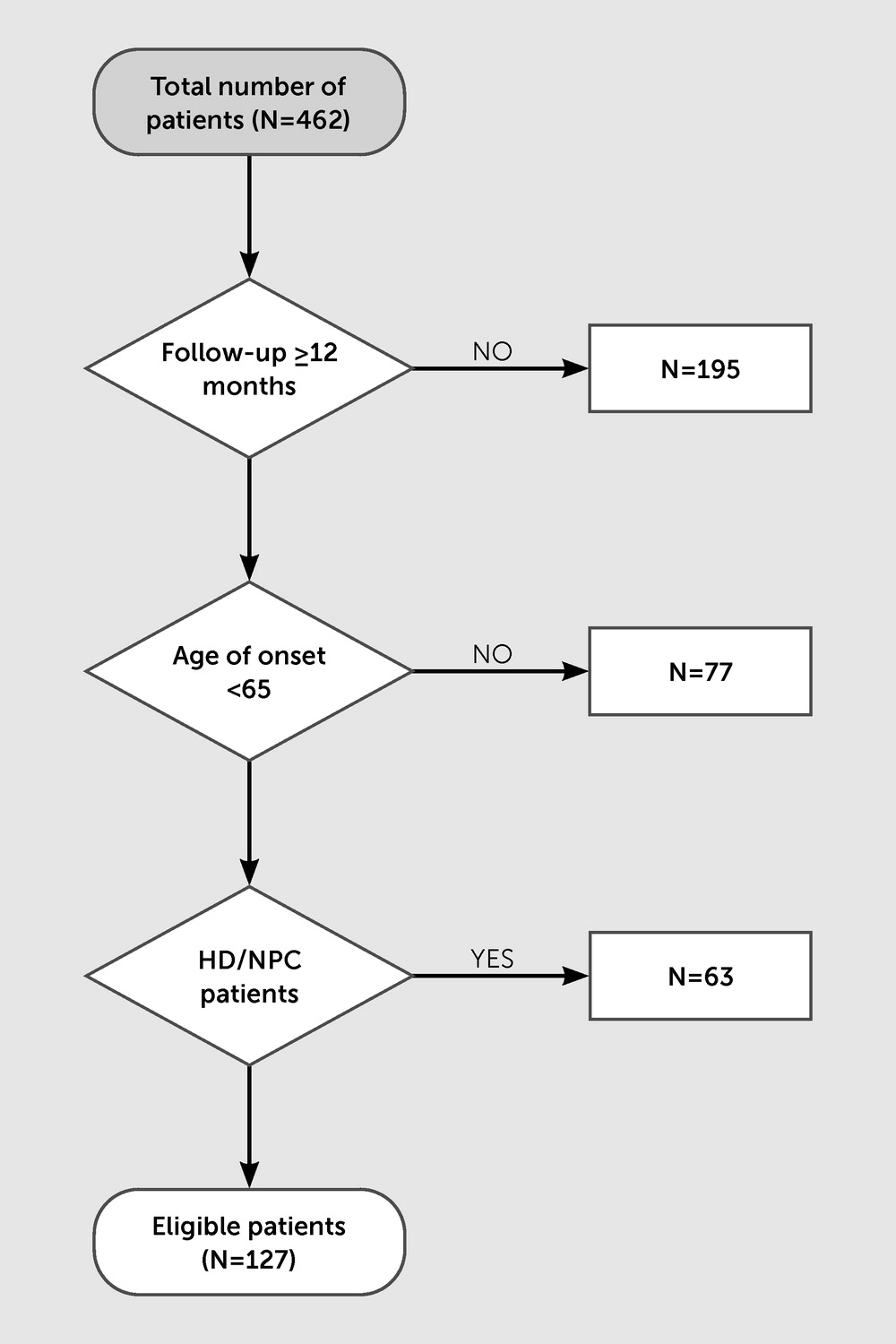
Study Design and Participants
Demographic and Clinical Variables
Diagnostic Information
Diagnostic Outcomes
Statistical Analysis
RESULTS
Baseline Demographic and Clinical Characteristics
| Characteristic | No change (N=78) | Change (N=49) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age at onset (years) | 49.7 | 8.8 | 50.7 | 8.2 |
| Age at admission (years) | 52.4 | 9.4 | 53.7 | 8.4 |
| Time until initial diagnosis (years) | 3.2 | 2.1 | 3 | 2.1 |
| Months of follow-up | 35.3 | 25 | 47.5 | 31.2 |
| Overall delay to final diagnosis (years) | 3.1 | 2 | 5.7 | 3.3 |
| Neuropsychiatry Unit Cognitive Assessment Tool score | 65.4 | 18.2 | 74.9 | 12.5 |
| N | % | N | % | |
| Male | 48 | 61.5 | 34 | 69.4 |
| Hypertension | 12 | 15.4 | 15 | 30.6 |
| Diabetes mellitus | 6 | 7.7 | 9 | 18.4 |
| Dyslipidemia | 33 | 42.3 | 18 | 36.7 |
| Smoker | 18 | 23.1 | 13 | 26.5 |
| Alcohol consumption | 23 | 29.5 | 16 | 32.7 |
| Tertiary education | 24 | 32.0 | 15 | 32.6 |
| Family history of dementia | 37 | 48.1 | 10 | 20.8 |
Diagnostic Change
| Initial diagnoses | Final diagnoses | Total | |||||
|---|---|---|---|---|---|---|---|
| AD-type dementia | FTD | VaD/mixed AD-VaD | Mild cognitive impairment | Primary psychiatric disease | Other | ||
| AD-type dementia | 0 | 0 | 1 | 0 | 1 | 3 | 5 |
| FTD | 2 | 1b | 0 | 1 | 7 | 5 | 16 |
| VaD | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Mild cognitive impairment | 0 | 0 | 0 | 0 | 1 | 5 | 6 |
| Primary psychiatric disease | 0 | 3 | 0 | 0 | 0 | 3 | 6 |
| Other | 1 | 2 | 0 | 2 | 2 | 7 | 14 |
| Total | 4 | 6 | 1 | 3 | 12 | 23 | 49 |
Comparison of Patients With No Change in Diagnosis With Those With a Diagnostic Change
Categories of Diagnostic Change
Within-category diagnostic change.
Between-category diagnostic change.
| Patient | Age at symptom onset (years) | Sex | Clinical presentation | Neuroimaging results at first admission | Psychiatric history | Initial diagnosis | Final diagnosis | Reason for change | Months of follow-up | Number of visits |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 48 | Male | Fluctuating memory impairment, apathy, and irritability | No abnormalities detected | Nil | Fronto-striatal dementia NOS | No progressive neurological or neurodegenerative disorder | No evidence of progression | 24 | 2 |
| Patient 2 | 54 | Female | Cognitive impairment, psychotic symptoms, and agitation | Atrophy in frontal and temporal regions; moderate hypoperfusion bilaterally involving all lobes | Long-standing history of mood difficulties; 1-year prior experienced delirium and psychosis | AD | Schizoaffective disorder | Relative functional stability/no evidence of progressive dementia | 84 | 2 |
| Patient 3 | NR | Male | STM impairment and gait disturbance | Marked atrophy and moderately severe atrophy of frontal and temporal regions | Chronic history of treatment-refractory schizophrenia | bvFTD | Paranoid schizophrenia | Improvement of cognitive impairment/lack of progression | 50 | 3 |
| Patient 4 | 50 | Male | Disinhibition | Severe atrophy of frontotemporal lobes and hypoperfusion | BPAD in the past 8 years | bvFTD | BPAD | No significant progression on serial imaging >5 years | 115 | 13 |
| Patient 5 | 36 | Male | Functional decline and STM impairment | Bifrontal sulcal prominence and prefrontal, frontal, and anterior parietal cortical hypoperfusion | Chronic schizophrenia | bvFTD | Schizophrenia | Stability of cognition on serial neuropsychology reports and MRI scans | 58 | 11 |
| Patient 6 | 32 | Male | Inappropriate behavior, amotivation, and specific dietary preferences | Moderate generalized atrophy and mild hypoperfusion of anterior temporal poles | Schizophrenia from age 22 | bvFTD | Schizophrenia | Improvement of behavioral and cognitive problems | 42 | 8 |
| Patient 7 | 42 | Male | Poor concentration, parkinsonism | No abnormality detected | BPAD diagnosed at age 20 | bvFTD | BPAD | Lack of progressive changes on neuroimaging | 16 | 2 |
| Patient 8 | 50 | Female | Inattention, cognitive slowing, reduced problem solving, and parkinsonism | Mild hypoperfusion in the temporal and parietal lobe | Postnatal depression, OCD behaviors, and delusional disorder | bvFTD | No progressive dementing disorder | Improvement on cognitive testing; parkinsonism resolved following cessation of antipsychotics | 16 | 3 |
| Patient 9 | 53 | Female | Symptoms of anxiety and somatic complaints | Moderate volume loss and hypoperfusion of frontal and parietal lobes | Chronic schizophrenia | bvFTD | Schizophrenia | Stable imaging; improvement on neuropsychology assessments | 12 | 3 |
| Patient 10 | 51 | Male | STM impairment and inappropriate behavior | Mild atrophy and extensive hypoperfusion bilaterally in parietal, temporal, and frontal lobes | Nil | VaD | Factitious disorder | Inconsistency between functioning and reported level of disability | 47 | 2 |
| Patient 11 | 60 | Female | STM impairment and anxiety | Atrophy most prominent in parietal regions and hypoperfusion in parietal and temporal lobes | Ongoing symptoms of dysthymia and anxiety | Mild cognitive impairment due to cerebrovascular disease | Depression | Lack of progression | 17 | 4 |
| Patient 12 | 52 | Male | STM impairment, apathy, limited insight, and attentional decline | Global atrophy and frontotemporal hypoperfusion | BPAD from age 22 | Dementia NOS | BPAD | Presentation consistent with long-standing BPAD | 18 | 2 |
| Patient 13 | 44 | Male | STM and attentional problems | No marked abnormalities | Experienced depressive symptoms 3 years prior | Dementia NOS | Schizophrenia | Lack of deterioration | 48 | 3 |
| Patient 14 | 56 | Male | Disinhibition, aggressiveness | Mild hypoperfusion in left parietal, anteriortemporal, and cerebellar lobes | Nil | No diagnosis | Dementing disorder NOS | Slowly progressing illness; behavioral disturbance; significant impairments in executive function; and visuospatial abilities | 94 | 2 |
| Patient 15 | 38 | Male | Significant memory and concentration impairment | Temporal hypoperfusion | Depression diagnosed 3 years prior | Depressive episode in remission | Dementia NOS | Clinical presentation and imaging results consistent with a progressive condition | 60 | 3 |
| Patient 16 | 51 | Female | STM problems and social withdrawal | Reduction in volume of frontal and temporal lobes bilaterally | Experienced psychotic symptoms 4 years prior | Late-onset psychosis | bvFTD | Deterioration in mental state | 12 | 2 |
| Patient 17 | 48 | Male | Disinhibition, apathy, and poor self-care | Cerebral volume at thelower limit of age-appropriate range | Depression diagnosed 2 years prior | Depression | bvFTD | Presentation and imaging results strongly suggestive of bvFTD | 30 | 4 |
| Patient 18 | 59 | Female | Cognitive impairment and depressive symptoms | Caudate atrophy | Experienced depressive symptoms | Depression | HD | Genetics (43 CAG repeats in HTT gene) | 65 | 1 |
| Patient 19 | 58 | Female | Treatment-resistant depression | Mild hypometabolism offrontal and temporal lobes | Diagnosed with depression for the past 10 years | Depression | bvFTD | Deterioration in cognition and imaging results suggestive of bvFTD | 72 | 6 |
DISCUSSION
Limitations
CONCLUSIONS
Footnote
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

