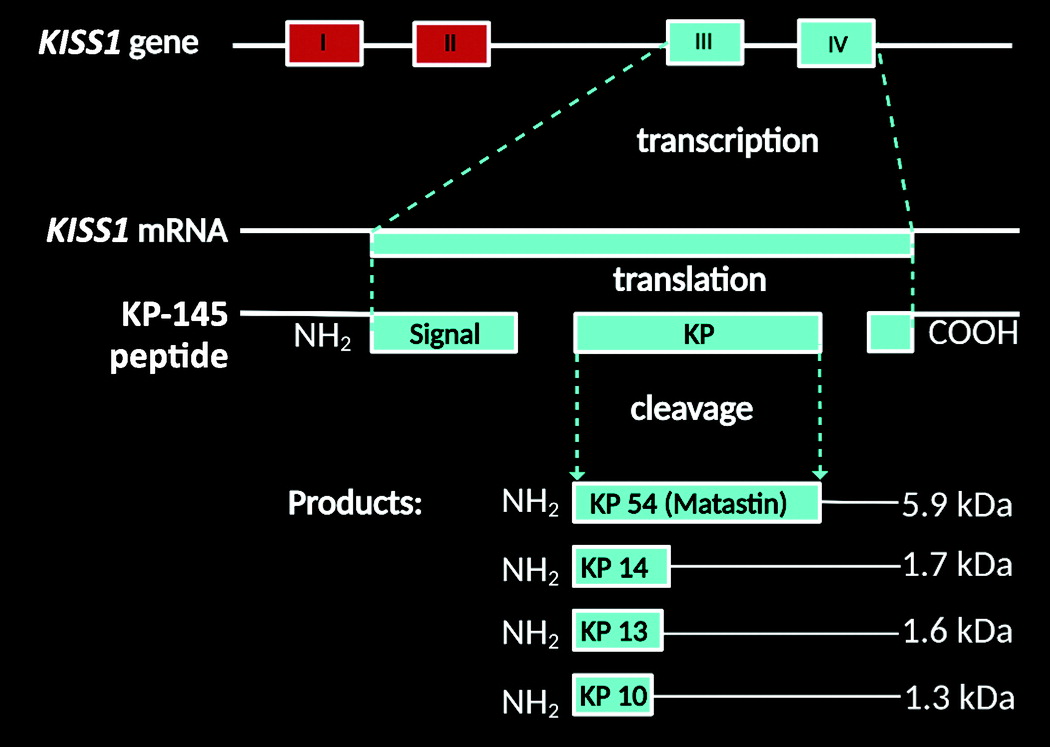
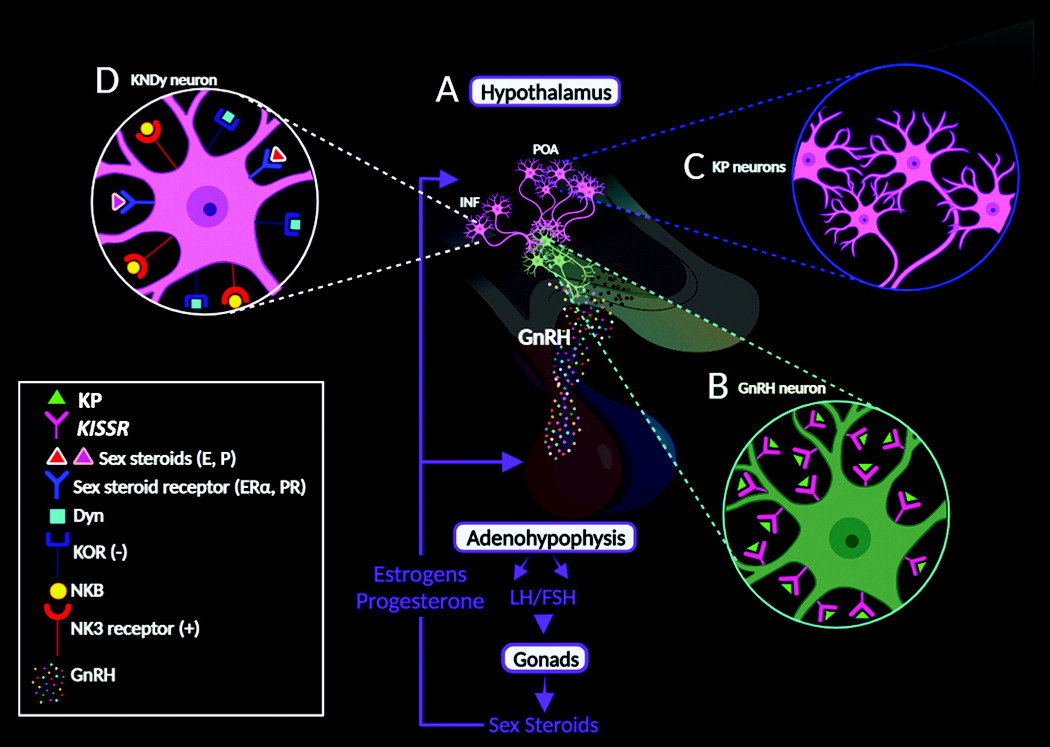
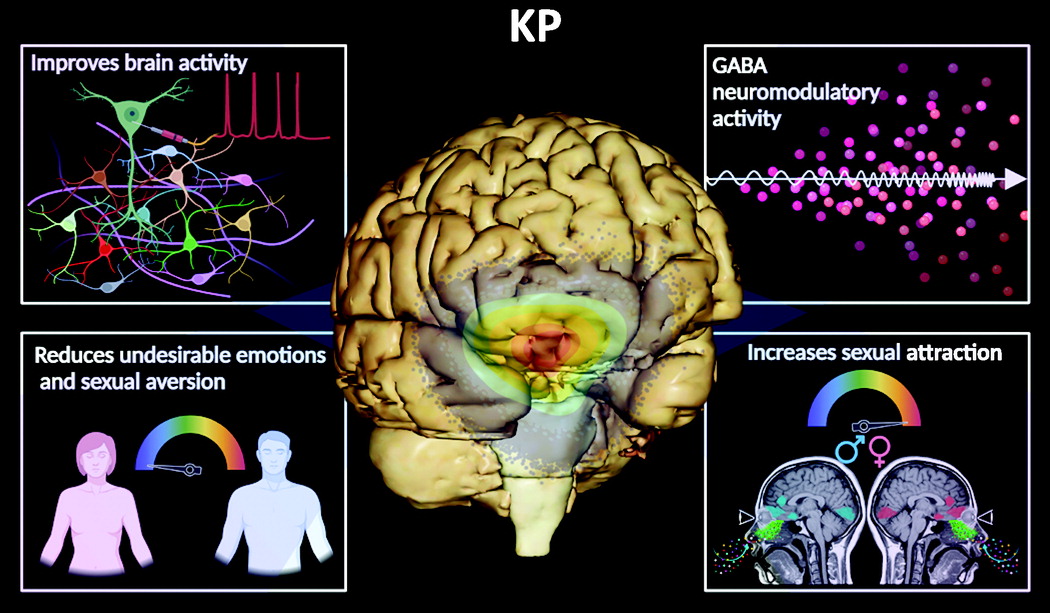
Habenula.
The habenula is recognized as a phylogenetically preserved diencephalic-paired neuroanatomical structure that is present in virtually all vertebrates (
52). The mammalian habenula comprises two important cellular groups or subnuclei: the medial habenula (MHb) and the lateral habenula (LHb). The medial component fibers (dopaminergic) connect with the interpeduncular nucleus, while the lateral cell group fibers (serotonergic) project to the ventral tegmental area (VTA) and raphe, respectively (
52). In humans, the habenula has an approximate diameter of 5–9 mm and a total volume of 30–36 mm
3 (
53).
The habenula is an important component of the emotion centers of the brain, contributing to the modulation of a wide repertoire of emotions, such as fear, reward, anxiety, and depression (
54,
55). Its neuroanatomical circuits include inputs from the limbic system, including the basal ganglia, as well as outputs to the midbrain, releasing dopamine (from the pars compacta of the substantia nigra and VTA) and serotonin (from the median and dorsal raphe nuclei, respectively). Thus, the habenula is involved in two major neuromodulatory monoaminergic pathways of the midbrain: the dopaminergic and serotoninergic systems (
54,
55). The habenula appears to function as a processing center for emotional and aversive responses, including aversively motivated learning and emotional decision making (
55–
58). In addition, it appears to have an important modulatory role in the perception of pain and certain mechanisms of analgesia (
59).
Emotional decision-making processes are associated with important physiological elements of reproduction (i.e., sex steroids and stress hormones) (
36,
40,
41). Habenular inputs from sex centers in the hypothalamic region potentiate key hormonal regulators via the HPG axis and KP circuitry (
58). Sex-hormone alterations during menopausal and pre- to postpartum transitions may lead to symptoms of depression and altered emotional processing (
60). Disturbances of the habenula may contribute (in part) to psychiatric conditions, such as addiction, attention-deficit hyperactivity disorder, major depression, and schizophrenia (
55,
61–
64). Moreover, results from a human brain study (postmortem histological tissues) revealed less neurons in the habenula and decreased volumes of both MHb and LHb in persons diagnosed with major depression or bipolar disorder (
65).
There are no significant sex differences in the structural anatomy of the habenula in humans. However, there are some important sexually dimorphic traits documented in the literature. For example, animal studies have revealed molecular dimorphisms in the expression of neurotransmitters, neuropeptides, and other neuroactive substances (e.g., glutamate, vasopressin, and tachykinins) (
58). Additionally, the habenula exhibits functional sexual dimorphisms in response to stress, including sex differences in metabolic processes and neural activity (
66–
68). In humans, the sex differences in habenular stress responses are connected with increased susceptibility to stress-related disorders (i.e., anxiety and depression), as well as fluctuating sex steroids (
69–
71). Predictably, the habenula and KP have been implicated in the modulatory mechanisms of anxiety, fear, reward, and mood regulation (
18,
19,
22,
51,
58,
72).
The LHb has a strategic anatomical location in the CNS, with pathways joining the forebrain to the ventral midbrain and hindbrain regions (
73). Some investigators have described the LHb as the “antireward center” of the brain (
74). Animal studies have revealed that it prevents behaviors leading to negative reward (i.e., punishment); however, it reinforces behaviors associated with positive reward (
75,
76). Therefore, the LHb regulates the mechanisms of motivated behaviors and decision making (
55). Social behaviors (e.g., avoidance, fighting, mating, and parenting) are important elements for communication in social animals (humans included), thereby critical for survival. These behaviors are regulated by neural circuits involving neuroanatomical structures (e.g., the habenula, amygdala, and prefrontal cortex), collectively referred to as the social behavior network (SBN), further integrating social decision making (
77). The cellular components of the SBN are significantly influenced by sex steroids (estrogens, progesterone, and testosterone) and certain neuropeptides (i.e., KP, GnRH, and Neuropeptide Y) required for the regulation of social behaviors (
77–
82).
Amygdala.
KP and its cognate
KISS1R gene are expressed in other key emotional structures of the limbic system, including the amygdala, in varied animal species (i.e., rodents) and humans (
20). The sex differences in the amygdalar size appear to be ambiguous, with some studies indicating larger volumes in females and others reporting larger volumes in males (
83–
86). Despite these inconsistencies, the average volumetric size of the human amygdala ranges between 1.24 cm
3 and 1.63 cm
3 (
87).
In addition to its roles in fear and anxiety, the amygdala also has an important role in reproduction, intervening over the release of gonadotrophic hormone, thus regulating essential mechanisms of reproductive physiology (
88). Aside from its canonical involvement in fear, the regulation of reproductive and social behaviors in a sexually dimorphic manner has become a new center of attention in neurophysiology (
24,
89). In humans, the peripheral administration of KP enhances functional MRI (fMRI) amygdalar activity in response to sexual and nonsexual contextual images among men (
19). Similarly, men receiving peripheral intravenous KP injections exhibited higher fMRI-resting connectivity in the amygdala-cingulate circuit, indicating enhanced sexual and emotional processing (
20).
Recently, the amygdala has been presented as an extrahypothalamic center, regulating body energy and metabolic homeostatic processes (
90–
92). There is an intimate connection between energy homeostasis and reproductive functions (
90). Sex hormones are important metabolic modulators, capable of controlling foundational elements of energy homeostasis. Sex steroid dysregulation may lead to mental illnesses and eating disorders, such as anorexia nervosa, bulimia nervosa, and binge eating disorder (
93–
96).