Cotard’s delusion, or the presence of nihilistic beliefs that may extend to the conclusion that one is dead, is associated with a range of neurological and psychiatric disorders (
1,
2). Although the mechanism underlying this delusion remains speculative, some reports have proposed misinterpretation of an impaired sensory input from a right-hemisphere localization as an underlying mechanism (
3). This is a case report on postictal Cotard’s delusion in a patient with subacute encephalopathy with seizures in alcoholism (SESA). SESA is a rare neurological syndrome with focal neurological deficits. The clinical course of this patient and the imaging findings support and clarify the proposed mechanism for this unusual delusional belief.
Case Report
A 69-year-old right-handed man with severe alcohol use disorder and thiamine deficiency was hospitalized with recurrent seizures followed by alterations in mental status and left-sided sensory loss with neglect. On admission, the patient vehemently complained that his left side had died and could not be used. Past medical history included a small left parasagittal frontal meningioma, laryngeal cancer (treated 17 years previously), pulmonary emboli, hepatitis C, and cervical and lumbar spondylosis. His social history included a 17-year incarceration followed by 8 years of homelessness and alcohol dependence.
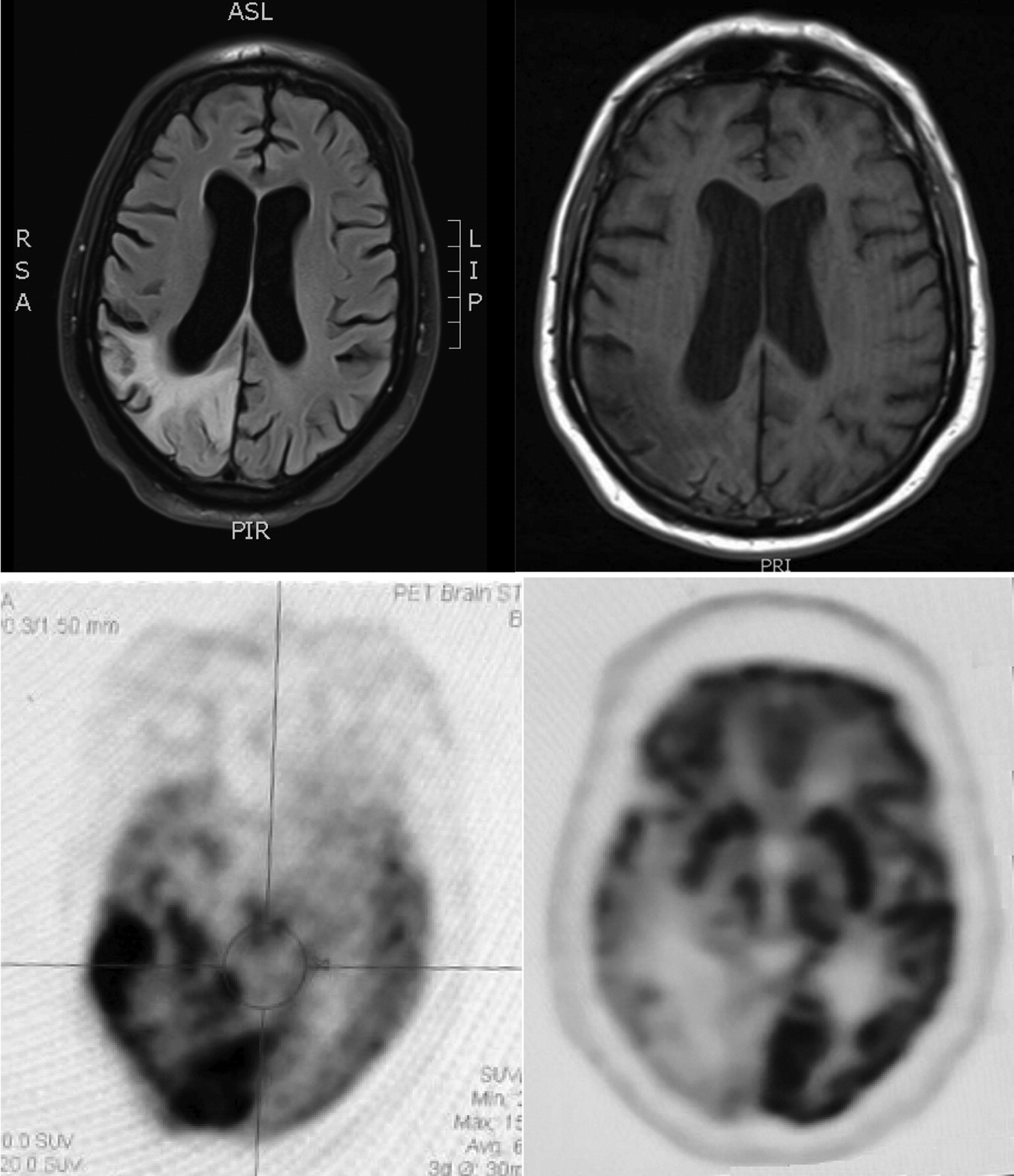
The man had four prior hospitalizations with similar episodes (i.e., recurrent seizures, alterations in mental status, and sensory loss with neglect) dating back 14 months. Each of these episodes began with apparent seizures described as shaking or clonic movements on his left side, followed by postictal confusion with an inability to complete sentences, left-sided visual loss, and left-sided weakness. Admission and nursing records from the most recent hospitalization also indicated initial agitation with complaints that his left side was dead. He had an extensive evaluation for vascular, infectious, and autoimmune etiologies that included unremarkable cerebrospinal fluid results. Magnetic resonance imaging (MRI) scans showed T2/fluid-attenuated inversion recovery (FLAIR) signal hyperintensity involving the right-hemisphere parietal, occipital, posterior frontal, and posterior temporal lobes but not in typical vascular territory (see
Figure 1). Corresponding fluorodeoxyglucose positron emission tomography (FDG-PET) scans showed hypermetabolic activity involving the same right-hemisphere regions (see
Figure 1). Electroencephalography (EEG) revealed that the patient had frequent periodic lateralized epileptiform discharges (PLEDs) that were maximal at O2 and occurred every 1 to 2 seconds. This was confirmed with continuous EEG with video showing intermittently recurring PLEDs every 1.5 to 3 seconds over the right occipital region. Repeating the continuous EEG with video captured two seizures that began with lateral head movements to the left that were followed by left arm clonic movements and onset of rhythmic spike-wave discharges on EEG from the bilateral temporo-occipital regions. Neuropsychological testing noted prominent verbal perseverations. He was impaired in verbal expression, repetition, and naming; however, his performance greatly improved when he was allowed to write his responses. Over the course of several days, as the patient’s symptoms mostly resolved, there were neuroimaging changes in the right parietal lobe; the MRI hyperintensity revealed volume loss, and the FDG-PET scans showed hypometabolism (see
Figure 1).
On examination, the patient was a disheveled, thin male who was inattentive and easily distractable, but he was oriented to person, place, and written date. He appeared agitated and complained throughout the examination that his left side was no longer living. Examination of the patient showed that his language was characterized by short phrases or answers with repetition and word-finding difficulty, yet he could speak grammatically correct sentences. Comprehension testing was compromised by his marked perseveration and intrusions from previous tasks. Naming was impaired; he could not name most objects that were presented visually, although he indicated recognition by describing them or mimicking their use. The patient was unable to read words that were spelled aloud, yet he could write intelligibly. His memory of the nature or sounds of the tested words was intact. The rest of the neurological examination included cranial nerve testing that showed a left hemianopia field loss, a reflex examination that was normal, and testing of motor function that was intact in the right-side upper and lower extremities. He did not follow commands for motor testing of the left side of his body, claiming that his limbs were dead while simultaneously moving them and lifting them up against gravity. Perception of light touch and pain was decreased in his left arm and leg, and he showed extinction to double simultaneous stimulation on the left side. Similar to previous admissions, his MRI scans showed an area of T2/FLAIR hyperintensity within the right parietal, occipital, and temporal lobes.
The overall assessment was that prolonged postictal Cotard’s delusion affected the left side of his body and that this was due to recurrent seizures consequent to antiepileptic medication nonadherence and SESA syndrome. The Cotard’s delusion of death of his left side gradually resolved along with improvements in his mental status and neuroimaging results. His seizures were initially controlled with levetiracetam and later with brivaracetam, and he was eventually discharged to a rehabilitation facility.
Discussion
This case report describes a patient with left-sided Cotard’s delusion due to SESA, which is a rare neurological syndrome that was originally reported in 1981 (
4). The patient’s right temporoparietal-occipital region had PLEDs on EEG, an increased T2/FLAIR signal on MRI scans, and hypermetabolism on FDG-PET scans. His complaint of left-sided Cotard’s delusion gradually subsided with stabilization and antiepileptic therapy. Right-hemisphere lesions, possibly affecting the superior parietal cortex, may be implicated in the pathophysiology of Cotard’s delusion through the impaired integration of sensory input and affective recognition of body ownership (
3,
5). During the postictal period, he also had perseverative verbal behavior with word-finding difficulty and possible optic aphasia, in that he had difficulty naming single letters when they were presented visually.
Cotard’s delusion, or the false belief of self-negation, is the misbelief that either part of one’s body or the entire person is deteriorating, destroyed, or dead. Although the most common cause of Cotard’s delusion is psychotic depression, it may result from a number of neurological disorders, including neurosyphilis, herpetic viral encephalitis, arteriovenous malformation, multiple sclerosis, Parkinson’s disease, brain neoplasm, migraine, traumatic brain injury, stroke, and epilepsy (
1,
2). Investigators have reported Cotard’s delusion of death in a patient with a unilateral right-hemisphere lesion that involved both depersonalization/derealization and delusional misidentifications of people and places (
3). They suggested that Cotard’s delusion may involve a two-step process of alterations in self-view or perception followed by the fixed, negative conclusion of deterioration or death.
This patient had Cotard’s delusion due to SESA, a distinct disorder that can occur among individuals with chronic alcohol dependence. SESA manifests with acute or subacute transient neurological deficits, focal seizures, and PLEDs on EEG on the side contralateral to the focal seizures (
6–
9). This condition differs from other complications of chronic alcohol use in that it is not clearly related to withdrawal and tends to recur with noncompliance with antiepileptic medications (
10,
11). Focal nonepileptic status epilepticus may account for the encephalopathy, focal neurological deficits, reversible diffusion-weighted MRI abnormalities, and focal hyperperfusion or FDG-PET hypermetabolism (
6); however, these changes may also be associated with intermittent postictal PLEDs (
9,
12–
14). Essentially, SESA involves a failure to return to baseline for a time after focal seizures that is associated with ongoing EEG changes along an ictal-interictal continuum (
6). The underlying mechanism of SESA and PLEDs is unclear but may be related to the mechanism underlying posterior reversible encephalopathy syndrome (
8).
Cotard’s delusion is often linked to right parietal, and possibly frontal and temporal, cortico-subcortical strokes or ischemia with left-sided sensory deficits including hemisensory loss and spatial neglect (
2,
3,
15–
17). Most neuroimaging studies of Cotard’s syndrome have noted abnormalities in the nondominant frontal and temporal lobes, and occasionally, the parietal lobes (
18). Some investigators, however, have reported Cotard’s delusion from left parietal strokes and cerebral ischemia localized in the left temporoparietal region (
2,
19). One review found that most neurological cases of Cotard’s delusion were accompanied by epilepsy (
18), probably due to an irritative focus in the right frontal and temporal lobes (
20), and postictal Cotard’s delusion has been reported in focal epilepsy patients (
21). Cotard’s delusion is related to other predominantly right parietal syndromes involving lack of familiarity or acceptance of part or all of one’s body, such as asomatognosia (i.e., loss of knowledge or awareness of body parts), somatoparaphrenia (i.e., the denial of ownership of a limb or one-half of the body) (
22), and depersonalization or derealization (
3).
The case reported here suggests that Cotard’s delusion can result when epilepsy-related impairments in somatic sensations fail to elicit the familiarity of ownership of a part of or the entire body. There is an overlap between nihilistic delusions of one’s body and one’s existence, and patients with Cotard’s delusion may misinterpret the meaning or source of impaired somatic or internal sensations (
23). The affective recognition of body ownership appears to depend on feedback sensations concerning one’s body or one’s internal body states and their integration into a body schema in the right superior parietal cortex (
24). This case enhances understanding of the mechanism underlying Cotard’s delusion and its neurological causes and may serve to elicit more research into a phenomenon that transcends neurology and psychiatry.