The brain accounts for only approximately 2% of total body weight. However, it consumes about 20% of the body’s energy, among the highest of all organs (
1). Brain energy metabolism (neuroenergetics) is an essential process for neural function and higher brain functions, such as memory and cognition (
6). The neuroenergetic requirements to sustain neural and brain function depend primarily on glucose consumption via glycolysis or mitochondrial respiration processes via oxidative phosphorylation. Glycolysis does not require oxygen, occurs in the cytosol, and results in two adenosine triphosphate (ATP) molecules. Mitochondrial respiration requires oxygen and generates a much higher energy yield of 30–36 ATP molecules (
1,
7,
8).
Glucose and oxygen are the primary energy substrates for the brain (
8). However, under specific circumstances (e.g., breastfeeding, adult ketosis, and diabetes), ketone bodies (KBs), lactate, and pyruvate act as alternative substrates for neurons (
1). These monocarboxylate substrates can sustain normal brain activity (e.g., neuronal function and synaptic activity) during glucose deprivation (
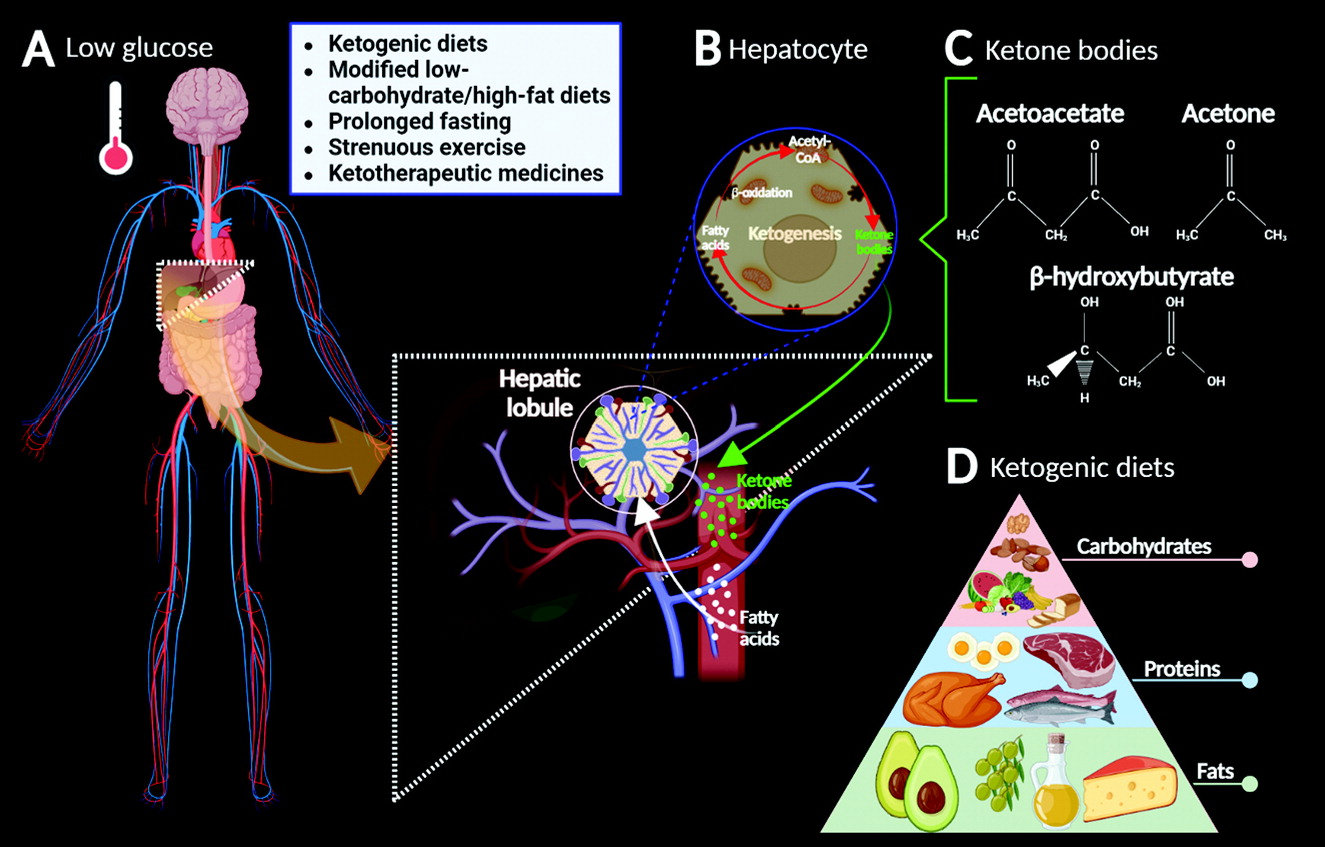
9). However, when systemic blood glucose levels drop, low endogenous carbohydrate levels cannot meet the body’s energy requirements, causing ketogenesis (
2,
3) (
Figure 1A–B).
KBs such as acetoacetate, acetone, and β-hydroxybutyrate can substitute for glucose as the primary source of energy for the body, particularly in the heart and the brain (
2) (
Figure 1C). Neonatally, KBs act as precursors for production of biological molecules, such as fats (especially cholesterol) and amino acids (
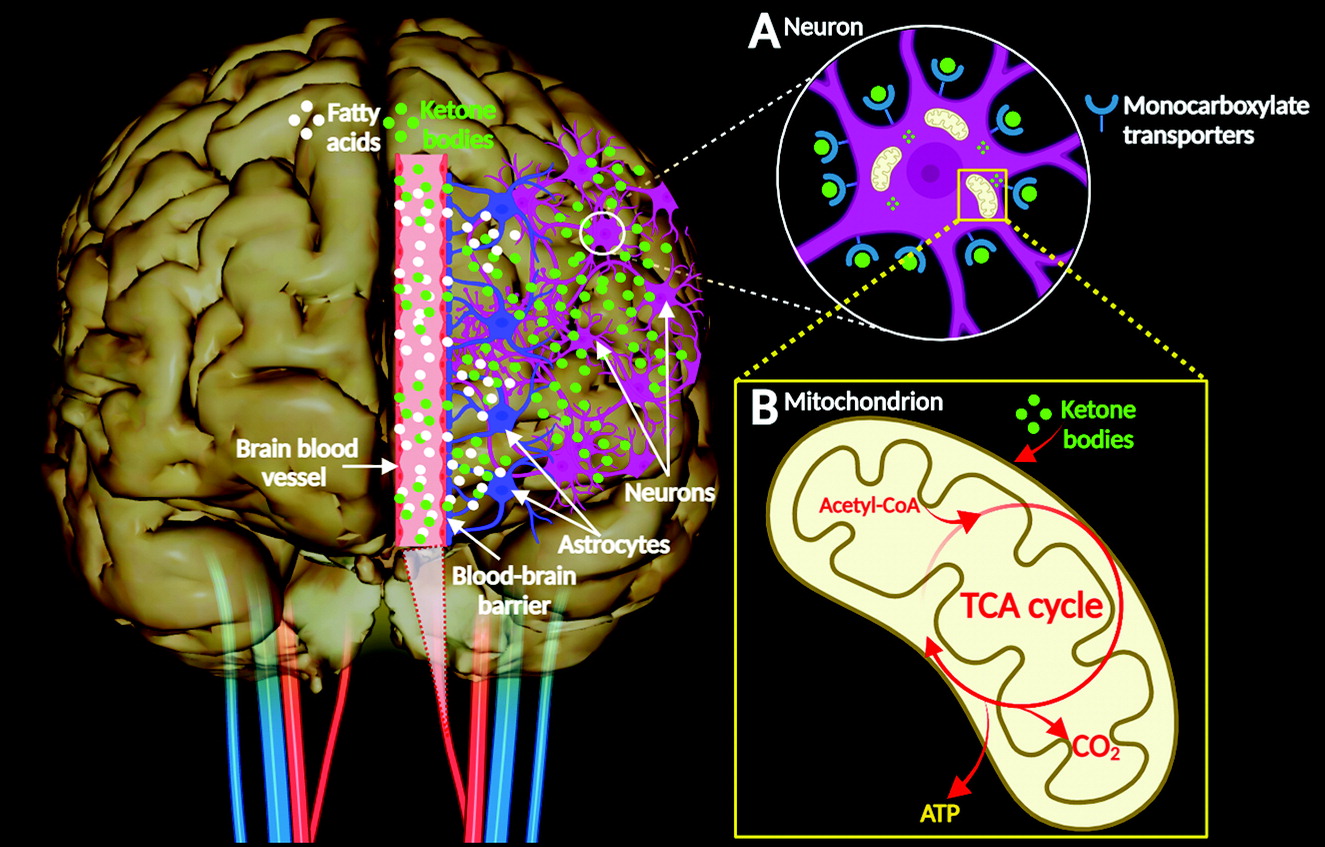
10). KBs enter nerve cells via monocarboxylate transporters and enter the mitochondrial metabolic pathway, resulting in the production of ATP (
Figure 2A–B). In the central nervous system (CNS), brain cells can use KBs as respiratory substrates for oxidative metabolic processes (
4). KB metabolic activity in the brain is regulated by the permeability of the blood-brain barrier, which depends on the abundance of cerebral monocarboxylate transporters, brain enzymatic processes, and other factors (e.g., diet, fasting, and exercise) (
10).
Ketogenic diets (KDs) are high-fat, high-protein, low-carbohydrate diets that result in the modulation of glycemia, elevated fatty acid levels, and relative caloric restriction (
2,
3) (
Figure 1D). Nutritionally, such diets increase the production of KBs, a process called ketosis (
2). However, ketosis can also occur in people consuming a low-calorie diet or a modified low-carbohydrate, high-fat diet and in individuals undergoing prolonged fasting periods and strenuous exercise (although the last two do not induce nutritional ketosis) (
11,
12). In addition, ketotherapeutic medicines (e.g., medium-chain triglycerides and ketone esters) are bioenergetic supplements that can induce ketosis and regulate energy metabolism (
12). These interventions are considered safe and well-tolerated treatments, potentially serving as excellent metabolic alternatives that prevent, slow, halt, or even reverse the development of some neurodegenerative diseases (
13,
14) (
Figure 1A–D).
Effects of Ketone Bodies in Neurological Diseases
KBs can provide neuronal protection during conditions of glucose deficiency, such as hypoglycemia (
1). Thus, multiple reports have confirmed some potential therapeutic benefits of KDs for various neurological conditions (
15–
18). A meta-analysis of 170 animal studies concluded that KDs provide multiple benefits involving aspects of epigenetics; neurotransmitter function, neuroinflammation, neuroprotection, and neuroplasticity; nociception; signaling pathways and synaptic transmission; and vascular supply (
18). Similarly, a systematic study summarizing trials of therapeutic use of KDs in traumatic brain injury (TBI) and other neurological disturbances (including aggressive brain tumors, ischemic stroke, and status epilepticus) concluded that KDs can be supportive in clinical management (
2). Neurological conditions with preliminary evidence showing the benefits of KDs are discussed below.
Traumatic Brain Injury
TBI is linked to a significant increase (∼85%) in the number of monocarboxylate transporter channels that facilitate the transport of KBs into neurons and is accompanied by a surge of β-hydroxybutyrate–metabolizing enzymes in these cells (
19–
22). These findings suggest that upon injury, the brain shifts to the energetic pathway involving the metabolism of KBs (
2). In line with this concept, the β-hydroxybutyrate components of KBs have two well-known neuroprotective properties: supporting the biochemical reconstruction of the respiratory chain and providing at least some energy from KB metabolism when the first complex of the respiratory chain via ATP-sensitive potassium channels is disrupted (
21,
22).
Results from rodent studies of TBI indicate that KDs improve cerebral metabolism, neuroprotection, and behavioral outcomes; protect myelin-forming oligodendrocytes; and reduce axonal damage while mitigating cerebral edema and apoptosis (
23–
26). A preliminary clinical trial revealed that a KD could be effective and feasible in adults with TBI (
26). However, clinical trial data supporting the widespread use of ancillary KDs in the clinical management of TBI remain limited (
27).
Epilepsy
KDs have been associated with positive outcomes in patients with medication-resistant epilepsy and febrile infection–related epilepsy syndrome (
3,
28,
29). KDs may be effective in treating infants and children with medication-resistant epilepsy by increasing levels of KBs, which in turn mechanistically contribute (at least in part) to seizure control, possibly due to anticonvulsant effects (
30–
32). Some of the anticonvulsant characteristics of KBs result from the amplification of brain messengers and neuroactive substances, such as gamma-aminobutyric acid, agmatine, and monoamines, thereby reducing neuronal hypersensitivity (
33). Anticonvulsive effects also result from the regulation of glutamate, possibly by altering the behavior of vesicular glutamate transporters, regulation of the neuronal membrane potential via ATP-sensitive potassium channels (activated during conditions of low ATP), and optimization of the tricarboxylic acid cycle and the electron transport chain cellular energy systems (
34).
Alzheimer’s Disease
The success of ketogenic therapies in epilepsy has increased interest in testing their use for other neurological disorders, such as Alzheimer’s disease (AD) (
12). Comorbid AD and epilepsy is being increasingly recognized in people of advanced age. Patients with comorbid epilepsy and AD may experience seizures and epileptiform discharges at any stage of AD (
35). In AD, mitochondrial dysfunction and decreased respiratory chain function alter processing of the amyloid precursor protein, which leads to increased production and deposition of beta-amyloid fragments in the brain (
36). In addition, high-sugar diets are linked to increased deposition of beta-amyloid fragments, suggesting that the brain’s insulin resistance may contribute to AD (
37,
38).
The synthesis of KBs may lead to specific neurological benefits, including reducing inflammatory and apoptotic mediators and improving mitochondrial functionality (
39). Furthermore, KBs generated from KD consumption decrease deposition and plaque formation of beta-amyloid fragments by reversing beta-amyloid neurotoxicity. Thus, KDs (including low-carbohydrate diets) might be useful in the clinical management of AD (
40).
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder of the CNS, affecting over 1% of people over 60 years of age (
41,
42). In addition to reductions in dopamine synthesis, the pathogenesis of PD seems to involve other contributing factors, such as abnormal glucose metabolism in the brain, inflammation in the CNS, mitochondrial dysfunction, and metabolic disturbances (
42,
43). Approximately 50%–80% of patients with a PD diagnosis also have impaired carbohydrate metabolism. In addition, peripheral insulin resistance, which is typically linked to brain insulin signaling and neuronal bioenergetic issues, is often seen in early PD (
43,
44).
Two important factors should be considered regarding KDs as a treatment for PD. First, high-carbohydrate, low-fat diets facilitate the availability of tyrosine, a dopamine precursor, in the cerebrospinal fluid, thereby increasing brain dopamine (insulin induced) (
45). This change improves one of the most important pathophysiologic hallmarks of PD, deficits in the neurotransmitter dopamine. Second, increased KBs (via KDs) may improve mitochondrial oxidative phosphorylation in the brain and bolster energy metabolism in central and peripheral neurons through mechanistic stimulation of mitochondrial biogenesis (
22,
46). These changes may contribute to attenuating the substantia nigra and frontal cortex deficits in respiratory chain complex I activity that have been reported in patients with PD (
42,
43,
47).
Levodopa (L-DOPA) is considered the primary medication for PD (
48). L-DOPA ameliorates PD motor symptoms but does not seem to have any neuroprotective effects (
49) and, paradoxically, may promote aggregation of alpha-synuclein (via the metabolite 5-S-cysteinyldopamine), inducing oxidative stress that can further deplete dopamine in the brain (
50). Some studies indicate that KDs improve the bioavailability of L-DOPA (
49,
51). Other studies suggest that simultaneous use of L-DOPA and a KD may halt the progression of PD symptoms (
49,
52,
53).
Animal and human studies indicate that KDs and ketotherapeutic supplements have other benefits, including protection of dopaminergic neurons from degeneration and improvements in motor function (
22,
51,
54). In a recent randomized controlled trial, 47 patients with PD were assigned to either a KD (high-fat diet) group or a low-fat diet group for 8 weeks. Both groups demonstrated significantly improved motor and nonmotor skills. However, the KD group showed greater improvements in nonmotor symptoms. In addition, the KD was confirmed to be a safe and reasonable intervention for patients with PD (
55). Although these results are promising, more investigation is required (
56).
Gliomas
Gliomas are highly heterogeneous brain tumors, and glioblastoma is the most aggressive type of glioma in adults. Glioblastomas have an extremely poor prognosis of approximately 12–15 months from the time of diagnosis, and the 5-year survival rate is below 5%. These tumors are characterized by a poor response to treatment (
57–
59). Glioma cells survive mainly on glucose and cannot function without it, suggesting that KDs could potentiate apoptosis. In a clinical trial, ketosis occurred in patients with glioblastoma who consumed a liquid KD 2 weeks before beginning chemoradiation therapy. These results suggested that a KD had some therapeutic effects and was both feasible and safe in combination with standard chemoradiation therapy (
59). In addition, a recent systematic analysis indicated that KDs are beneficial for patients with malignant gliomas, mostly based on higher rates of survival (
60).
Migraine
Migraine is a chronic disease, resulting from both genetic and environmental factors. Two systematic studies reported therapeutic potential for KDs, with one study noting that KD interventions reduced the number of attacks and the intensity of headaches among participants with migraine (
2,
61).
Multiple Sclerosis
Multiple sclerosis (MS) is a neurodegenerative and inflammatory condition of the CNS with an autoimmune origin (
2,
62). A growing body of evidence suggests that a KD is beneficial for those with MS and is both safe and feasible (
63–
65). Additionally, the clinical evidence suggests that patients with relapsing MS who follow a KD over a 6-month period typically experienced neuroprotective and desirable disease-modifying effects, such as reduced fatigue, depression, neurological disability, and adipose-related inflammation (
65).
Limitations of KETOGENIC Diets
KD and ketotherapeutic interventions have some potential adverse effects and contraindications. Adults who consume a KD often report weight loss; gastrointestinal side effects, such as constipation, diarrhea, nausea, and vomiting; and a transient increase in lipids. Although rare, headaches, abdominal pain, irregular menstruation, drowsiness, nephrolithiasis, and pancreatitis also have been associated with KDs (
2,
66,
67). Consuming low-carbohydrate diets may also lead to reduced appetite and suppressed hunger. Older people, especially those with dementia, typically have a lack of appetite and dysphagia. The use of KDs in such individuals may cause them to omit meals, resulting in malnutrition and other nutrient deficiencies, which may worsen their condition (
68). In a study of patients with PD, KDs were associated with periodic tremors or stiffness, increased irritability, and exacerbated hunger or thirst (
55). Long-term use of KDs may also cause more serious side effects, including hyperuricemia, proteinuria, and metabolic acidosis, especially among individuals with coexisting diabetes and inadequate insulin management. In addition, increased aminotransferase and other liver enzyme activity has been reported, suggesting a temporary increase in hepatic enzyme activity possibly associated with a KD-induced hypercholesteremia (
67,
69).
Finally, there is the so-called “keto flu,” a cluster of symptoms and side effects that appear within the first few weeks of KD initiation. Symptoms may include “brain fog,” headache, nausea, dizziness, fatigue, gastrointestinal discomfort, constipation, arrhythmias, or low energy (
70). Overall, these symptoms are transient and seem to be mild (
2,
70). Serious adverse effects of KDs seem rare and usually result from a lack of or inadequate clinical supervision. Thus, use of KDs and ketotherapeutic approaches should be individualized for each patient (
2).
Conclusions
In summary, nutritional regimens (KDs and ketotherapeutic supplements) that generate increased KBs in plasma and the brain (ketosis) appear to have substantial potential to improve neuronal processes, such as mitochondrial metabolism, cell signaling, and neurotransmitter function. Additionally, KDs can reduce oxidative stress, inflammation, and toxicity, which can increase neural network stability and thereby improve cognitive function. There is a growing body of evidence supporting the benefits of KBs for some neurological conditions. However, there remains a lack of data from randomized, blinded trials in large populations and relevant subpopulations to determine the feasibility, sustainability, and long-term effects of ketotherapeutic interventions, either alone or as adjuvant treatments for CNS disorders.