Glucagon-like peptide 1 (GLP-1) is an incretin, a gut-derived hormone released from enteroendocrine L-cells (distributed along the entire gastrointestinal tract) after food intake, leading to the secretion of insulin from pancreatic beta cells in a nutrient-dependent manner (
12). GLP-1 is also secreted from alpha cells in the pancreas and multiple regions in the central nervous system. There are two bioactive forms of this peptide hormone in the bloodstream: GLP-1-(7–36)-NH
2, the more abundant form (approximately 80%), and GLP-1-(7–37), the less abundant form (approximately 20%). GLP-1 has a relatively short half-life of approximately 2–5 minutes once released into plasma. This is a result of (in part) the action of the enzyme dipeptidyl-peptidase-4 and GLP-1’s low molecular weight, which accelerates renal clearance (
5,
13).
Both GLP-1-(7–36)-NH
2 and GLP-1-(7–37) interact with a specific GLP-1 receptor, a class B G protein–coupled receptor (
2). GLP-1 receptors are widely expressed throughout multiple human tissues (e.g., muscles, bones, and adipose) and organs (e.g., gastrointestinal tract, pancreas, kidneys, lungs, heart, blood vessels, and brain) (
14,
15). GLP-1 receptors mediate the physiological actions of GLP-1, which include the regulation of glucose homeostasis (decreasing glucose secretion), appetite control (i.e., cravings and satiety), body weight management, metabolism, glycogen synthesis in skeletal muscle and liver, gastric peristalsis, lipid metabolism and modulation of fat absorption, and cardiovascular and neurologic function, among others (
2–
5).
The extensive anatomical distribution and range of physiological roles of GLP-1 suggest its important pharmacotherapeutic potential. GLP-1 receptor agonist therapies have been used mainly to treat type 2 diabetes mellitus and obesity (
3). More recently, new medications (e.g., semaglutide) have become popular because of their efficacy in reducing body weight in obese patients with and without diabetes (
16,
17). At present, there are seven GLP-1 receptor agonists approved as medications for type 2 diabetes, cardiovascular disease, and obesity: albiglutide, dulaglutide, exenatide, extended-release exenatide, liraglutide, lixisenatide, and semaglutide (
18). The Food and Drug Administration (FDA) approved the first GLP-1 receptor agonist medication, exenatide, in 2005. Semaglutide was the most recent, receiving FDA approval in 2019. Most of these medications are injected subcutaneously, but semaglutide can also be administered orally (typically once daily) (
18). Dulaglutide is indicated for hemoglobin A1C management in patients at high risk of cardiovascular disease events. Liraglutide (at a high dose) was approved for obesity management in patients over 12 years old with a body mass index above 30. In 2021, the FDA approved high-dose semaglutide injections for chronic weight management (
18). At present, GLP-1 receptor agonist pharmacotherapies are being evaluated for other clinical uses (
3). For example, they are promising neuroprotective agents, preventing neuroinflammation and supporting cognitive improvement (
19–
21).
GLP-1 in the Brain
GLP-1 and its analogs can readily cross the blood-brain barrier (BBB), where its neuroactive properties can benefit nervous tissue (
22). This neuropeptide is also synthesized in some brain regions, including hypothalamic nuclei, the nucleus tractus solitarius, and the caudal brainstem (
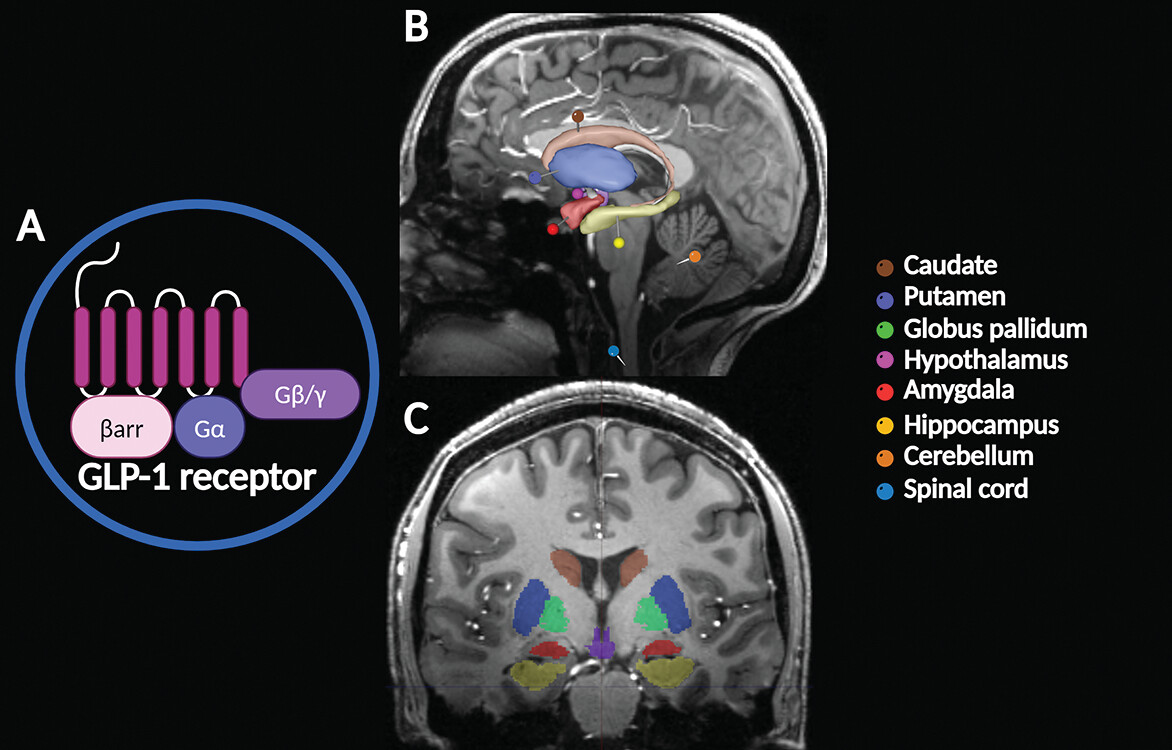
23). GLP-1 receptors are expressed in the caudate, putamen, globus pallidum, hypothalamus, amygdala, hippocampus, cerebellum, and spinal cord (
Figure 1) (
1). GLP-1 has important neurophysiological attributes, mediating neuronal processes such as mitochondrial function, protein aggregation, and synaptic plasticity (
9,
14,
22). In recent years, studies have described a central role for GLP-1 in regulating food intake, providing neuroprotection, decreasing neuroinflammation, amplifying signal transduction, and potentiating cognitive function by counteracting learning dysregulation (
3,
5,
24).
GLP-1’s ability to regulate food intake and appetite was originally linked to the midbrain (hypothalamic nuclei) and hindbrain (the nucleus tractus solitarius) (
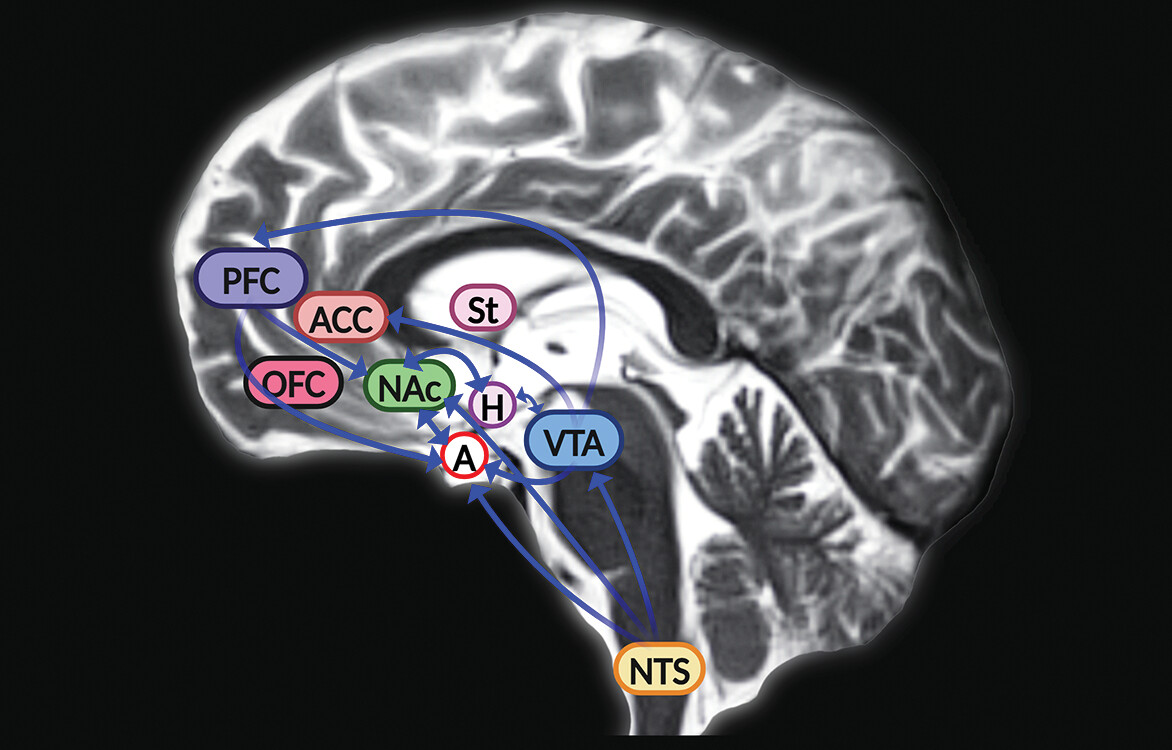
5). However, animal studies revealed that GLP-1 receptors are also expressed in mesolimbic regions of the brain such as the ventral tegmental area and nucleus accumbens, where it mediates (in part) the neural mechanisms of reward and motivation associated with food intake (
Figure 2) (
6–
9). In rodents, the GLP-1 receptor agonist exendin-4 induced anorexia and attenuated alcohol-mediated behaviors through activity in the mesolimbic reward system (
7,
25–
27). Interactions between GLP-1 and alcohol appear to involve a comprehensive neural circuit, encompassing limbic reward and motivation areas and hindbrain structures (
27). In the hindbrain, GLP-1 is synthesized in the neurons of the nucleus tractus solitarius, which project to the reward centers of the brain (
6). GLP-1 is a multifaceted neuropeptide that can modulate important behavioral and neurochemical physiological responses linked to alcohol use and the use of other drugs, such as nicotine, opioids, cocaine, and amphetamines (
28). GLP-1 analogs have the potential to help treat addictions by inhibiting dopamine release in the reward centers, consequently reducing withdrawal effects and relapses (
29). Taken together, evidence from preclinical and clinical studies suggests that the administration of GLP-1 analogs could become a future therapeutic approach to treat substance use disorders (
27,
28).
The dopaminergic pathways of the amygdala (among other limbic structures) mediate responses in the reward circuit of the mesolimbic system (
30). In the amygdala, dopamine signaling is increased during food ingestion, and GLP-1 is involved in the regulation of feeding behavior (
31). Animal studies have shown robust expression of GLP-1 receptors in the central portion and the capsular and lateral regions of the amygdala (
11,
32). In rodents, GLP-1 receptors appear to have an important function in feeding motivation, but whether these receptors are expressed in the human amygdala remains unclear (
11).
The central amygdala is a complex neuroanatomical region, with a mixed population of orexigenic and anorexigenic cell bodies (
11). It receives projections from the nucleus tractus solitarius (
11,
33). Thus, the activity of GLP-1 receptor agonist medications in the central amygdala are speculated to mediate (in part) the anorexigenic effects of these compounds. In addition, these medications may regulate mechanisms linked to emotional and motivational feeding (
11).
Anatomically and physiologically, astrocytes are among the most important components of the BBB. Astrocytes are central regulators of glucose metabolic processes in the brain (
34), and they play a key role in responses to acute ischemic stroke, altering the molecular structure of the BBB and secreting inflammatory chemicals (
35). GLP-1 receptors are also expressed in hypothalamic astrocytes (
34). Exedin-4 protects the BBB in acute ischemic stroke by decreasing inflammatory responses (
36). Recent clinical studies suggest that GLP-1 receptor agonist therapies can reduce the effects of stroke among individuals with type 2 diabetes (
37–
39).
Neurodegenerative conditions such as Alzheimer’s disease and Parkinson’s disease have been linked to type 2 diabetes and insulin dysregulation in the central and peripheral nervous systems (
40,
41). Brain insulin resistance can occur without peripheral insulin resistance, and both have been linked to cognitive decline (
42,
43). Furthermore, GLP-1 increases insulin signaling in the brain (
44). GLP-1 receptor expression in the brain supports hippocampal synaptic transmission and regulates cellular apoptosis, potentiating the regulation of neurological and cognitive functions (
1,
14,
45). GLP-1 receptor deficiency has been linked to a higher risk of seizures and neurodegenerative processes, whereas its overexpression promotes neuroprotection and improved cognition (
20,
46). GLP-1 receptor agonist compounds have shown relevant neurostimulatory and neuroprotective activity in animal and human trials. Some studies have indicated that their pleiotropic properties appear to ameliorate aging-related neurodegenerative diseases, particularly Alzheimer’s disease and Parkinson’s disease (
20,
44,
47,
48).
Many individuals living with severe mental illness (e.g., schizophrenia spectrum disorders, bipolar disorder, and major depressive disorder) rely on antipsychotic medications (
49). However, these therapies provide only limited symptomatic relief from a subset of symptoms and can cause side effects, with some medications increasing the risks of type 2 diabetes, metabolic syndrome, obesity, and cardiovascular diseases (
10,
50). In addition, antipsychotics do not target cognitive impairments, which typically accompany some mental illnesses (
21,
51,
52).
GLP-1 is a powerful brain-signaling molecule with potential as a neuropsychiatric pharmacotherapy (
21,
53). GLP-1 exerts neurostimulatory and neuromodulatory effects, regulating the release of several neurotransmitters, such as serotonin, dopamine, gamma-aminobutyric acid, and glutamate, which mediate (in part) important neurophysiological mechanisms linked to depression-related behaviors and those associated with other psychiatric disorders (
54). Results from preliminary studies in animals have indicated that GLP-1 receptor agonists can mediate microglial activity, prevent neuroinflammation, and help improve anxiety-related emotional behaviors (
55,
56).
Given the evidence thus far, administration of GLP-1 receptor agonists appears to support cognitive function and may improve mood symptoms among individuals with anxiety and depression (
20,
44,
47,
48,
53–
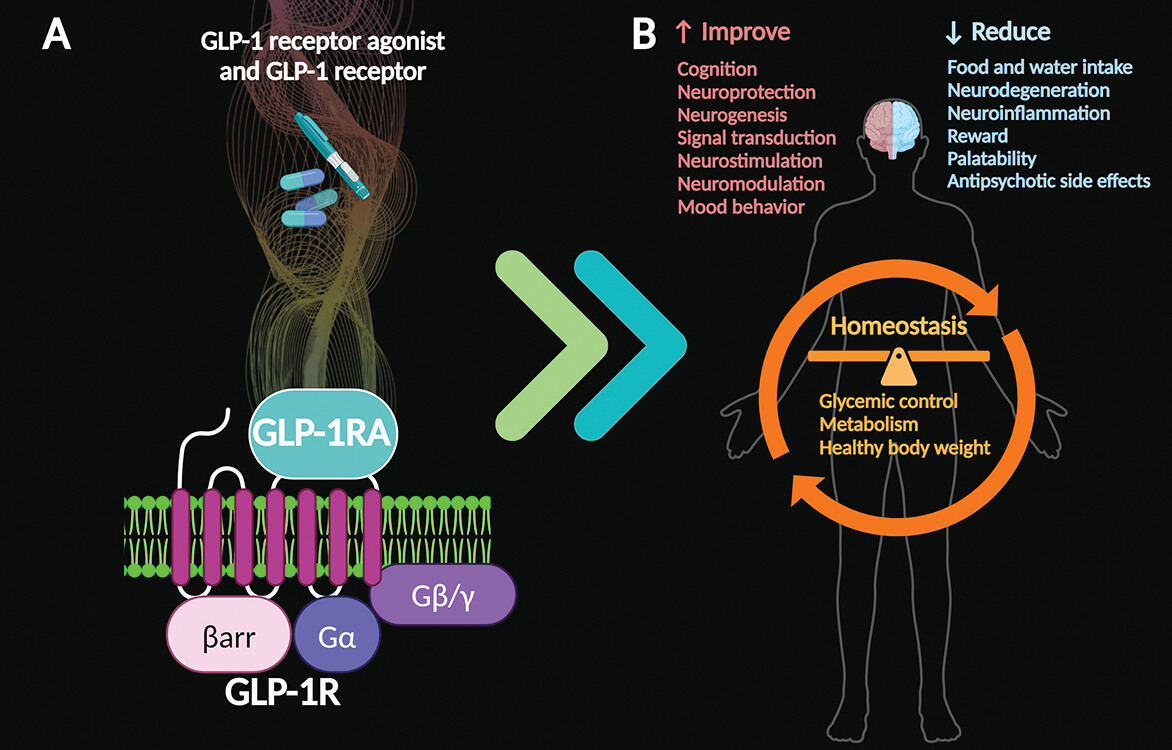
56). The proven systemic benefits of GLP receptor agonists can help reestablish overall homeostasis by balancing glycemic control, improving metabolism, and supporting healthy body weight (
3–
5,
21). Furthermore, GLP-1 receptor agonist therapies may help reverse or alleviate the metabolic side effects of antipsychotic medications (
Figure 3) (
21,
53).