Disorders of white matter are common in many clinical settings across the lifespan; cognitive dysfunction can frequently emerge and be so severe as to produce the syndrome of white matter dementia (
1,
2). Central to this concept is the notion that transdiagnostic clinical-pathological commonalities can be found across a broad spectrum of white matter disorders. Although these disorders are variously described in a highly divergent body of neurologic and psychiatric literature, a synthetic integration of findings shows that white matter pathology is present in a wide range of genetic, demyelinative, infectious, inflammatory, toxic, metabolic, traumatic, neoplastic, hydrocephalic, and vascular disorders (
1,
2). Integrative review of these categories leads to a convergence of evidence that cognitive decline not only occurs in these disorders but manifests with a distinct neurobehavioral profile reflecting the white matter involvement (
1,
2). Indeed, this work has increased appreciation that the cerebral cortex is not the only brain region associated with cognitive dysfunction or dementia (
1–
3). However, while the understanding of white matter-behavior relationships has substantially advanced in recent years, less attention has been devoted to the management of patients with impaired cognition related to white matter disorders. Given the high prevalence of these disorders and their potential for disabling cognitive decline (
1–
3), a focused effort to identify effective intervention is an important priority.
In this review, new perspectives on treatment and recovery are presented to demonstrate the remarkable capacity of white matter for restoration and plasticity. White matter can no longer be regarded as static and immutable, or as simply supporting the gray matter to which it is connected. In contrast, recent investigations clearly show that white matter is neurobiologically dynamic and continually engaged in the process of structural modification under the influence of environmental factors. Such changes in white matter are well established in the healthy brain, and recent evidence suggests that they can also occur in the presence of pathology. The reduction or reversal of structural white matter pathology with treatment suggests that cognitive improvement may plausibly be expected; data relevant to this question will also be described. A special focus on vascular white matter lesions will be developed in view of the cognitive impairment these lesions can produce and the risk they pose for subsequent dementia (
3). These insights therefore have important implications not only for the care of patients with white matter-associated cognitive impairment but also the prevention of dementia later in life.
Structural Changes With Current Treatment of White Matter Disorders
A useful starting point for this discussion is a consideration of currently available treatments for white matter disorders with respect to their capacity to improve the structure of white matter. Although relatively little study has been devoted to this question, preliminary evidence of salutary morphologic change after treatment is available. The advent of MRI and its companion technique, diffusion tensor imaging (DTI), has enabled detailed visualization of treatment-related improvement in white matter macrostructure or microstructure and encouraged thinking about the potential for treatment to effect still greater improvement as therapeutic knowledge expands.
All 10 categories of white matter disorder illustrate how damaged regions can benefit from targeted treatment (
4–
13) (
Table 1). Despite the markedly differing neuropathology of these disorders, treatment directed at the primary neuropathological process can, in some cases, effect observable improvement in white matter structure. These observations indicate that standard treatment of the various white matter disorders can be implemented with at least some expectation that structural benefit may occur. A consistent theme that emerges from this work is that recovery of white matter is far more likely when axons are preserved, as myelin can be restored if the axonal cylinders are intact (
14). This principle is clearly evident in leukoaraiosis (
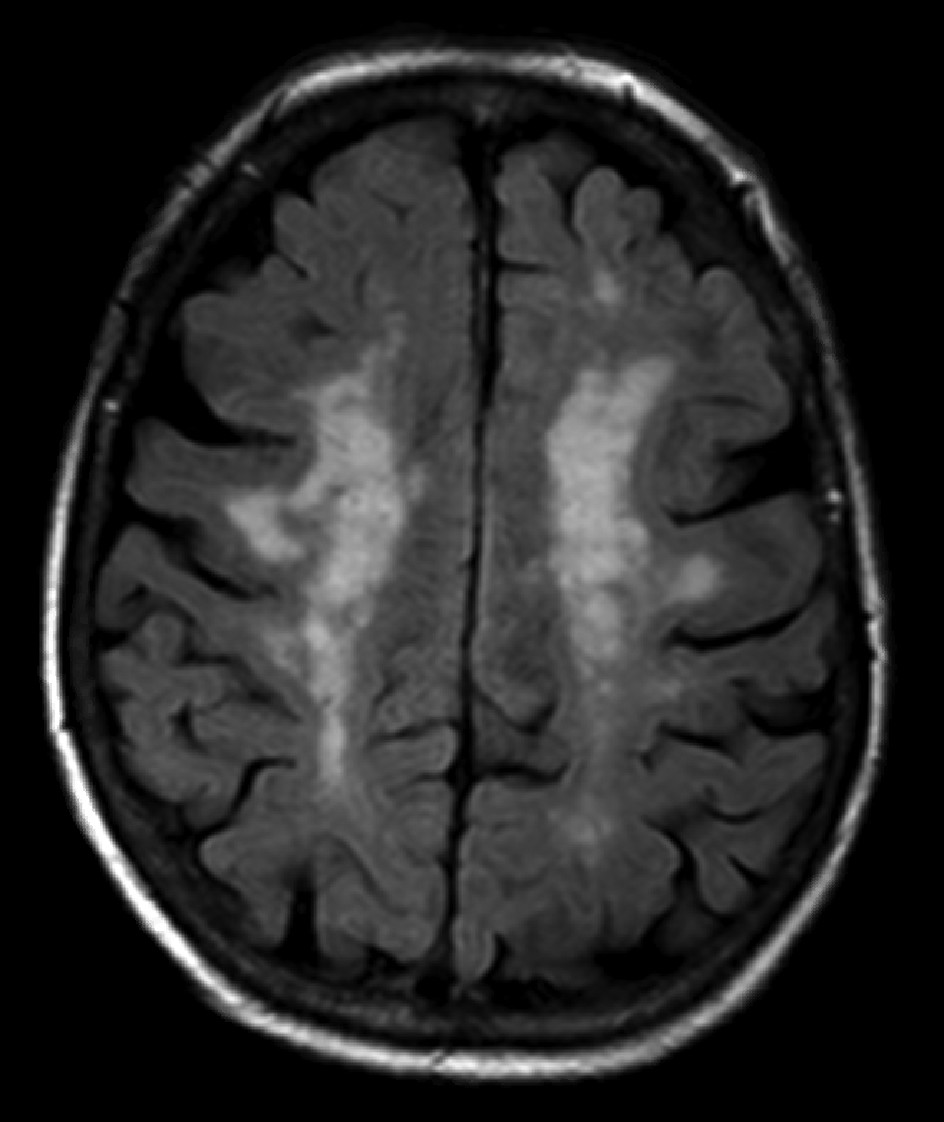
15), a term often used to describe the seemingly ubiquitous cerebral white matter hyperintensities on MRI scans of older people that are widely interpreted as ischemic in origin (
Figure 1). These lesions, characterized by ischemic demyelination and relative axonal preservation (
16), have been shown in an increasing number of studies to regress in a substantial number of patients with treatment of hypertension (
Table 1).
White Matter Restoration
The data reviewed above indicate that disordered white matter structure can, in some fashion, be restored by targeted treatment of the specific neuropathology involved. It is now appropriate to consider this observation in detail by returning to the problem of leukoaraiosis (
15), a disorder in which restoration of white matter can now be regarded as an outcome in some patients. In contrast to the widely held assumption that leukoaraiosis inexorably worsens over time, recent evidence has documented the remarkable finding that regression of ischemic white matter hyperintensities can occur in a substantial number of affected individuals over a period of 1–5 years (
13,
17–
20). Given the common experience of many clinicians that continual accumulation of leukoaraiosis is to be expected as people age, lesion regression is indeed surprising and has prompted some to query whether this observation may reflect measurement artifacts related to varying MRI technologies or inaccurate volume estimations. These issues have been addressed, however, by several studies that used rigorous MRI methodology with standardized assessments to improve the reliability of the findings (
13,
19,
20), and measurement artifacts appear unlikely. Al-Janabi and colleagues (
20), for example, followed 351 participants without dementia who were in the Alzheimer’s Disease Neuroimaging Initiative for 2 years and found leukoaraiosis regression in approximately 25% that was associated with improved memory and decreased brain atrophy. No treatment-related explanation for the resolution of leukoaraiosis in these individuals could be established, but resolution of reversible ischemic changes or a regenerative process in the white matter were considered most likely (
20).
Studies conducted with hypertensive adults found similar results, and the factor repeatedly identified as responsible for regression of leukoaraiosis was the treatment of hypertension (
17–
20). Most noteworthy in this regard is the large multicenter Systolic Blood Pressure Intervention Trial Memory and Cognition in Decreased Hypertension study. This study compared hypertensive adults with systolic blood pressure <120 mm Hg to adults with systolic blood pressure <140 mm Hg over 5 years, and found that intensive treatment reduced conversion to mild cognitive impairment and, in a subgroup of individuals who had MRI, produced a smaller increase in leukoaraiosis volume (
19). Dementia incidence was also lower in the intensive treatment group, although without reaching statistical significance (
19), but conversion from cognitively normal to mild cognitive impairment is arguably a more significant transition with respect to early treatment of cognitive dysfunction related to leukoaraiosis.
These observations are consistent with current thinking that leukoaraiosis is a common problem in older people that has a vascular origin, affects myelin but typically spares axons, and can be considered incomplete infarction from arteriolar narrowing that leads to reversible perivascular edema and ischemic demyelination (
16,
21). Whereas white matter hyperintensities in older people may occasionally represent other neuropathology, such as chronic plaques of multiple sclerosis (
2), the great majority are likely explained by leukoaraiosis. What is new in the clinical neurobiology of this disorder is that ischemic white matter lesions can regress over time in a substantial percentage of patients. The disappearance of the MRI white matter hyperintensities is most likely a result of reduction of the water content within the lesions of leukoaraiosis, with secondary improvement in tract structure and function. Regression of leukoaraiosis in 20%−40% of affected patients over a period of several years with adequate blood pressure control (
13,
17–
20) indicates that medical intervention can have observable benefit in a substantial number of older individuals. The mechanism of this effect is presumably due to reperfusion of white matter, and cognitive improvement may be observed in parallel with this process. A crucial implication of these findings is that dementia risk may also be reduced, as will be discussed below.
White Matter Plasticity
The example of leukoaraiosis, which demonstrates the novel phenomenon that restoration of ischemic white matter can occur with medical treatment or perhaps even spontaneously, implicates macrostructural lesions of white matter best seen on conventional MRI. To complement this development, evidence is rapidly accumulating that microstructural changes in the brain white matter may also be effected by the process of plasticity (
22). This concept, simply defined as the capacity of the brain to change with experience, applies to both gray and white matter (
23,
24) and is attracting much interest as a potentially powerful means of treating a broad range of neuropathological states. In the gray matter, plasticity may result from synaptogenesis, neurogenesis, and angiogenesis, all of which can lead to increased cortical thickness; treatment strategies based on these mechanisms are being investigated (
24). In the white matter, plasticity is essentially a capacity of myelin and refers to the enhancement of myelination with experience (
22,
23). White matter plasticity has been extensively studied in nonhuman animals; in humans, studies are beginning to appear in normal subjects and in persons with neurologic disease. The phenomenon appears to be relevant to the macrostructure of white matter tracts, and perhaps even more so to what has been termed the normal-appearing white matter (NAWM), meaning the white matter that is unremarkable on standard MRI but may harbor early pathology that is only seen by more advanced techniques such as DTI.
The essential feature of white matter plasticity is the phenomenon of activity-dependent myelination. This concept has been well established in nonhuman animal models, in which the formation of new myelin has been identified as a novel mechanistic basis of learning that complements learning that occurs in gray matter via traditional synaptic function (
22,
23). At its core, activity-dependent myelination describes the capacity of myelin to be laid down the distal axon as a function of proximal electrical activity (
22,
23). The process occurs because there are in fact synapses in the white matter that mediate the activation of myelination as a result of volleys of action potentials traversing the axon. In a significant development, the discovery of these synapses shows that synaptic connections exist not only in the gray matter, offering one more indication that white matter is a dynamic and malleable region of the brain with its own capacity for remodeling. The white matter synapses are axo-oligodendroglial and employ glutamate as they function to integrate the axon with the central nervous system glial cell that mediates the essential task of myelinating axons in the brain (
22,
23). This arrangement means that environmental experience, which serves to increase the electrical activity of axons, can influence myelination of distal axonal segments and confer an important source of neural circuit plasticity (
22,
23). The process is most robust in development but has been shown to occur in adulthood and old age as well (
22,
23).
Studies of white matter plasticity in cognitively normal individuals have found that the structure of white matter can be altered by a wide range of activities ranging from juggling and piano practice to exercise and memory training (
Box 1) (
23,
25). Remarkably, white matter changes can occur in as little as 2 hours, as shown in a study of young adults who were trained in a computer car-racing game (
26). Relatively few clinical studies of this phenomenon have been presented to evaluate this principle in disease states, but it appears that both white matter macrostructure and microstructure may benefit from targeted intervention. In individuals with Broca’s aphasia from a left hemisphere infarct, for example, Schlaug and colleagues (
27) found that melodic intonation therapy increased the size of the right arcuate fasciculus while improving language in the patients treated. At the level of the NAWM, poor readers who had reading instruction had significantly increased fractional anisotropy in the left anterior centrum semiovale that correlated with improvement in phonological decoding ability (
28). These preliminary data indicate that targeted intervention may activate white matter plasticity in disease states as well as in health.
Another recent development in clinical neuroscience that may inform the area of white matter plasticity is noninvasive brain stimulation. This approach posits that beneficial effects on brain structure can be induced by the application of magnetic or electrical stimulation to the cranium adjacent to areas of cerebral injury. Among the more studied modalities are transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Preliminary evidence from paretic stroke patients suggests that both TMS (
29) and tDCS (
30) can increase ipsilesional white matter integrity in parallel with improved motor function. Although the impact of noninvasive brain stimulation on cognitive function has been little studied, and it is unclear to what extent white matter can be impacted by modalities widely assumed to exert their primary effects on the cerebral cortex, noninvasive brain stimulation may prove useful in promoting white matter plasticity with clinically meaningful cognitive benefit.
Implications for the Prevention of Dementia
The evidence presented above for regression of leukoaraiosis and white matter plasticity suggest that medical intervention can have salutary effects on both the macrostructure and microstructure of white matter in the brain. These insights clearly imply that patient care can be improved by the application of routine, noninvasive, and inexpensive measures that target the white matter and, in some cases, improve cognition. In addition, however, the exciting possibility exists that these treatments may prove effective in dementia prevention.
With the steady accrual of information on the cognitive consequences of leukoaraiosis, it has become apparent that these lesions are not incidental, as was often assumed when MRI white matter hyperintensities were first noted in the 1980s. Indeed, among the many risks of leukoaraiosis is the development of dementia later in life. In this regard, the most recent data indicate that leukoaraiosis confers a 25% higher risk of Alzheimer’s disease and a 73% higher risk of vascular dementia (VaD) (
3). In parallel with the emergence of these data is accumulating evidence for a steady decline among industrialized countries in dementia incidence and prevalence over the past several decades (
29,
30). Factors contributing to these epidemiological trends include improved living conditions and social welfare, higher education levels, and better health care (
31,
32). The socioeconomic advantages present in developed countries permit many policies that overlap to a large extent with the measures discussed above that foster the health of brain white matter. To illustrate this point, a large population-based study from the Netherlands showed that older people examined in 2006 had fewer white matter lesions than demographically similar people studied in 1995 (
33). Although it is regrettable that all countries cannot share in the advantages enjoyed by the developed world, the principles illustrated by analysis of the data are vitally important, and their application to all countries is a critical task that promises to reduce the worldwide burden of dementia.
These considerations bear directly upon the etiopathogenesis of Alzheimer’s disease and VaD, the two most common late-life dementias. The new observations clearly indicate that genetics explains only a proportion of dementia; a heightened emphasis on the social determinants of brain health is warranted (
3,
29–
32). Although societal policies to address these social determinants are surely warranted, there is much that individual clinicians can do to avert or lower the risk of dementia by attention to common problems earlier in life, most notably vascular risk factors. This emphasis would most directly affect VaD, since both leukoaraiosis and VaD have a vascular origin, and it is highly plausible that the one is a precursor to the other (
2,
16).
With respect to Alzheimer’s disease, the myelin model of Bartzokis (
34) directly pertains to the question of pathogenesis by its postulation that the disease begins with breakdown in the white matter. According to this model, white matter in aging is vulnerable to pathology as result of volume loss, a process known as retrogenesis; Alzheimer’s disease pathology is a consequence of the accumulation of vascular and other changes that eventually lead to the deposition of amyloid plaques and neurofibrillary tangles in the cerebral cortex and hippocampus (
34). The myelin model of Alzheimer’s disease thus introduces the idea that neurodegeneration, typically regarded as a gray matter process, may also implicate white matter pathology, particularly early in pathogenesis (
34). Support for this idea comes from a study of 4,415 older people without dementia who were followed for 7 years after DTI and in whom lower fractional anisotropy and higher mean diffusivity in multiple tracts increased the risk of developing all-cause dementia and Alzheimer’s disease; a vascular mechanism was thought most likely (
35). Of considerable interest is that these microstructural white matter changes may benefit from hypertension control, as shown by the study of Wardlaw and colleagues (
13) that found reduced mean diffusivity of the NAWM in stroke patients who had larger reductions in blood pressure.
Although many details of pathogenetic links between white matter damage and Alzheimer’s disease pathology are yet to be elucidated, it is intriguing, for example, to consider recent postmortem findings documenting that midlife hypertension is associated with plaques and tangles late in life (
36). A wide variety of other environmental risk factors may be implicated in the pathogenesis of Alzheimer’s disease by virtue of their effects on white matter (
Box 2) (
37). Although more study is needed, these modifiable risks can all be plausibly seen as exerting their preventive effects because of this impact on white matter. It could well be, therefore, that a focus on the protection of white matter long before dementia is most likely to arise—in midlife and even before—may be transformative; interventions that could be effective would theoretically target both the macrostructure and microstructure of white matter. The repeated failure of anti-amyloid and anti-tau drugs in Alzheimer’s disease has been much discussed (
38), lending credence to the notion that cortical protein deposition may not be a useful therapeutic target and suggesting that investigation of an upstream process in the white matter may be more promising as a solution to this dreaded disease.
To summarize the case for dementia prevention broadly considered, current evidence suggests that 30%−35% of dementia could be prevented by attention to modifiable risk factors (
30). These estimates imply that Alzheimer’s disease, VaD, and perhaps other late-life dementias share common pathogenic antecedents. Indeed, because copathology of Alzheimer’s disease and VaD is the rule and not the exception in late-life dementia, a strong case can be made that maintenance of vascular health stands out as the primary preventive objective (
25,
37,
39). Other risks such as traumatic brain injury, depression, cognitive inactivity, and sleep dysfunction also merit consideration in this regard (
Box 2). The question does arise, however, concerning the remaining 65%−70% of late-life dementia that is not currently preventable; in these cases genetic factors assume more importance in pathogenesis (
39). Yet it is possible that even genetics may be amenable to environmental influences so that genetic risks for dementia can be mitigated. For example, a population-based prospective study found that life course factors—education, substantive work complexity, a social network, and leisure activities—reduced the risk of dementia to the extent that these factors overrode the genetic predisposition to Alzheimer’s disease associated with the apolipoprotein E-ε4 genotype (
40). As these life course factors operate substantially at the level of the brain white matter, perhaps dementia prevention can be significantly enhanced by the specific targeting of myelin plasticity as a means of impacting the early-life determinants of late-life cognitive function (
25,
26). Environmental intervention aimed at white matter may therefore offer the prospect of helping avert dementia even in people who are at genetic risk.