Association Between Migraine Comorbidity and Psychiatric Symptoms Among People With Newly Diagnosed Focal Epilepsy
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Participants
Questionnaires
Statistical Analyses
Results
Demographic Characteristics
| All participants (N=349) | Epilepsy patients without migraine (N=275) | Epilepsy patients with migraine (N=74) | pa | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | |
| Female | 209 | 59.9 | 153 | 55.6 | 56 | 75.7 | <0.001 |
| Race and/or ethnicityb | 0.69 | ||||||
| Unknown | 18 | 7.8 | 16 | 5.9 | 2 | 2.8 | |
| White | 274 | 78.5 | 216 | 78.5 | 60 | 81 | |
| Black or African American | 42 | 12 | 32 | 11.6 | 10 | 13.5 | |
| Asian | 12 | 3.4 | 10 | 3.6 | 2 | 2.7 | |
| American Indian or Alaska Native | 1 | 0.3 | 1 | 0.4 | 0 | 0 | |
| Hispanic or Latino | 29 | 8.3 | 23 | 8.4 | 6 | 8.1 | 0.89 |
| Highest level of education | 0.81 | ||||||
| Did not finish high school | 18 | 5.2 | 15 | 5.5 | 3 | 4.0 | |
| GED | 4 | 1.1 | 3 | 1.1 | 1 | 1.4 | |
| High school | 48 | 13.8 | 37 | 13.5 | 11 | 14.9 | |
| Associate’s degree | 38 | 10.9 | 32 | 11.6 | 6 | 8.1 | |
| Some college | 56 | 16.0 | 39 | 14.2 | 17 | 23.0 | |
| Bachelor’s degree | 108 | 30.9 | 85 | 30.1 | 23 | 31.1 | |
| Graduate school | 75 | 18.5 | 62 | 22.5 | 13 | 17.6 | |
| Level of employment | 0.067 | ||||||
| Full time vs. not full time | 219 | 62.8 | 182 | 66.2 | 37 | 50 | 0.011 |
| Part time | 39 | 11.2 | 29 | 10.5 | 10 | 13.5 | |
| Homemaker | 12 | 3.4 | 7 | 2.5 | 5 | 6.8 | |
| Student | 30 | 8.6 | 21 | 7.6 | 9 | 12.2 | |
| Unemployed | 45 | 12.9 | 32 | 11.6 | 13 | 17.6 | |
| Family history of seizures | 106 | 31.5 | 82 | 30.9 | 24 | 33.3 | 0.70 |
Headache Characteristics
Primary Outcomes
| Variable | Total number of participants with data available | All participants (N=349) | Epilepsy patients without migraine (N=275) | Epilepsy patients with migraine (N=74) | pb | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| CES-D score ≥16 | 341 | 86 | 25.3 | 61 | 22.7 | 25 | 35.2 | 0.031 |
| GAD-7 score ≥10 | 341 | 25 | 7.4 | 13 | 4.8 | 12 | 16.9 | <0.001 |
| C-SSRS ideation | 337 | 76 | 22.6 | 54 | 20.5 | 22 | 30.1 | 0.080 |
| C-SSRS behavior | 337 | 20 | 5.9 | 13 | 4.9 | 7 | 9.6 | 0.140 |
| C-SSRS ideation or behavior | 337 | 76 | 22.6 | 54 | 20.5 | 22 | 30.1 | 0.080 |
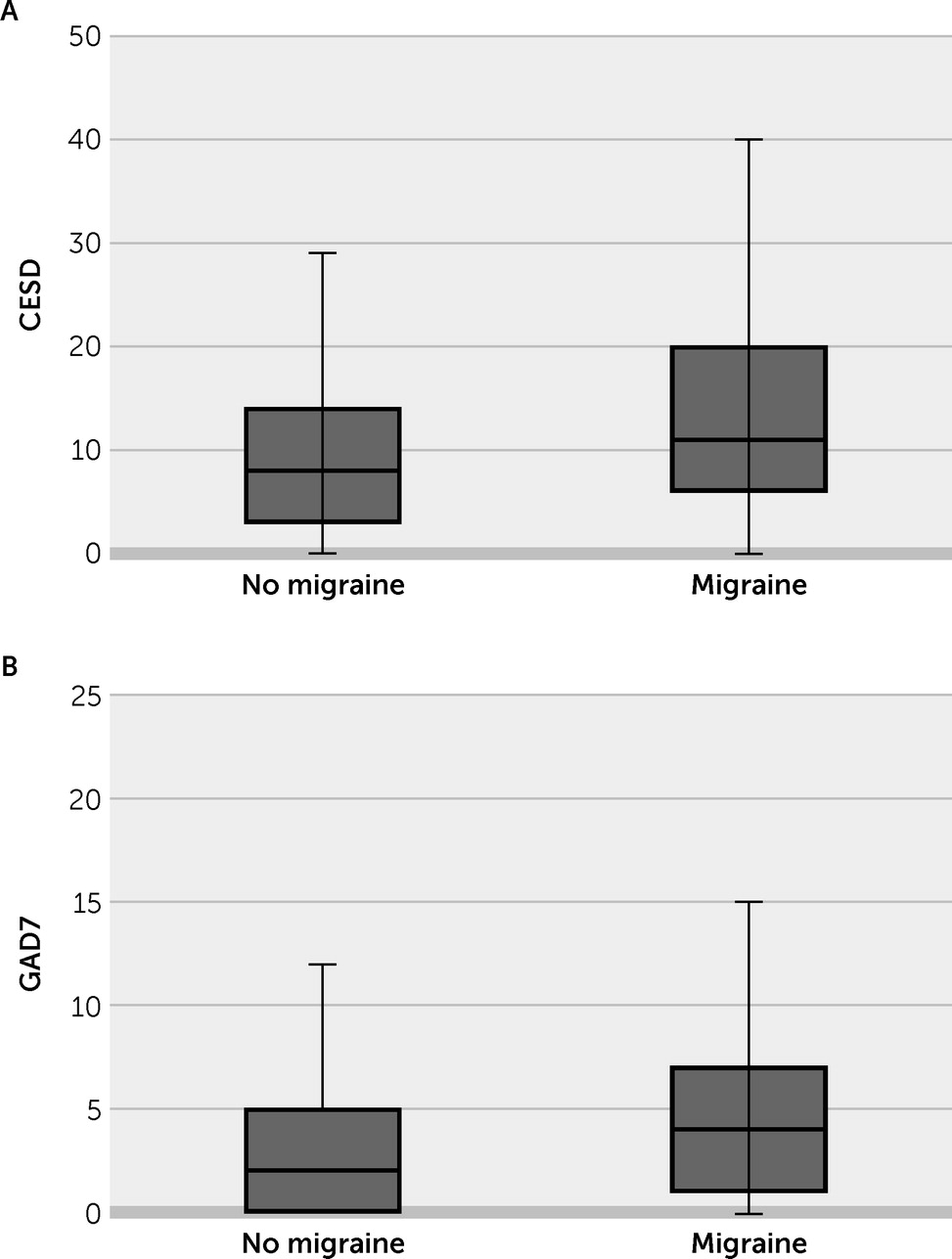
Discussion
Limitations
Future Studies
Conclusions
Footnotes
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

