Excessive impulsivity is a hallmark finding among people with many psychiatric conditions, including substance use and hyperactivity and attentional disorders (
1,
2). The specific neural circuits likely involve disrupted communications between frontal and subcortical and limbic brain structures, including the amygdala and nucleus accumbens, based on evidence ranging from the classical case of brain injuries in Phineas Gage to animal studies (
3,
4). Neuroimaging studies of human subjects with, and at risk for, substance use disorders support this hypothesis. For example, increased impulsivity and reduced functional connectivity and microstructural integrity of white matter in cortico-striatal pathways predispose individuals to the development of impulsivity-driven phenotypes spanning many behaviors (
5,
6). Here, we hypothesize that white matter integrity may be associated with normal between-subject variability in impulsivity measures. We tested this hypothesis with a large dataset of community-dwelling adults without psychiatric illness by mapping multiple domains of impulsivity using a self-reported questionnaire.
The Barratt Impulsiveness Scale–11 (BIS-11) is the most widely used assessment for impulsivity as a multidimensional behavior. The commonly used version consists of 30 questions that have been divided into three subdomains: attentional, motor, and nonplanning aspects of impulsiveness. The components of the BIS-11 have shown acceptable psychometric properties (
7,
8), test-retest reliability (
8), and clinical validity (
9–
11), raising an interesting question of whether some of these subcomponents can be mapped onto particular brain circuits. Therefore, a secondary aim here was to determine whether some of these impulsivity subdomains can be mapped onto specific white matter tracts.
Although it is an indirect measurement of white matter microstructure, fractional anisotropy (FA) from diffusion tensor imaging (DTI) has been extensively studied in its relationship to human behaviors, cognition, and health (
12), thought to reflect, in part, the degree of myelination, although the exact biophysics remain complex and not fully delineated (
13,
14). One previous study has examined the correlation between white matter and impulsivity among healthy community-dwelling individuals and found an interesting but seemingly paradoxical association in which higher FA in the prefrontal cortex-nucleus accumbens tract was associated with more impulsivity (
15). However, it is unlikely that any one tract alone underserves such a multifaceted and complex behavior in its entirety. This study did not report results from other white matter tracts, which also left open the question of whether different white matter tracts in the brain should be systematically examined, not just in relationship to the overall impulsivity score but also to different subdomains of the impulsivity construct.
Our study attempted to more comprehensively approach the question of white matter contributions to impulsivity using BIS-11 total and subdomain scores. We then sought to rank the strength of the association between each impulsivity measure and the major white matter tracts to identify the white matter tracts that most strongly contribute to specific impulsivity measures.
Discussion
Impulsivity is a complex behavior that has remained a challenge to study and understand operationally and neurobiologically. Barratt and colleagues (
7) attempted to define this construct with the BIS-11 questionnaire. Despite its widespread use in studies of normal behaviors, psychopathology, and neuropathology (
11,
26–
28), recent debates have challenged its validity and interpretability (
29,
30). However, genetic association studies using the BIS-11 have found evidence linking impulsiveness to serotonergic promoter region polymorphism (
31) and motor impulsiveness to monoamine oxidase A genotype (
32), providing validation support. With new technology to investigate the brain and its functions in vivo, we attempted to further map impulsiveness and the subdomains of this behavior onto structural connectivity in the brain of otherwise healthy adults.
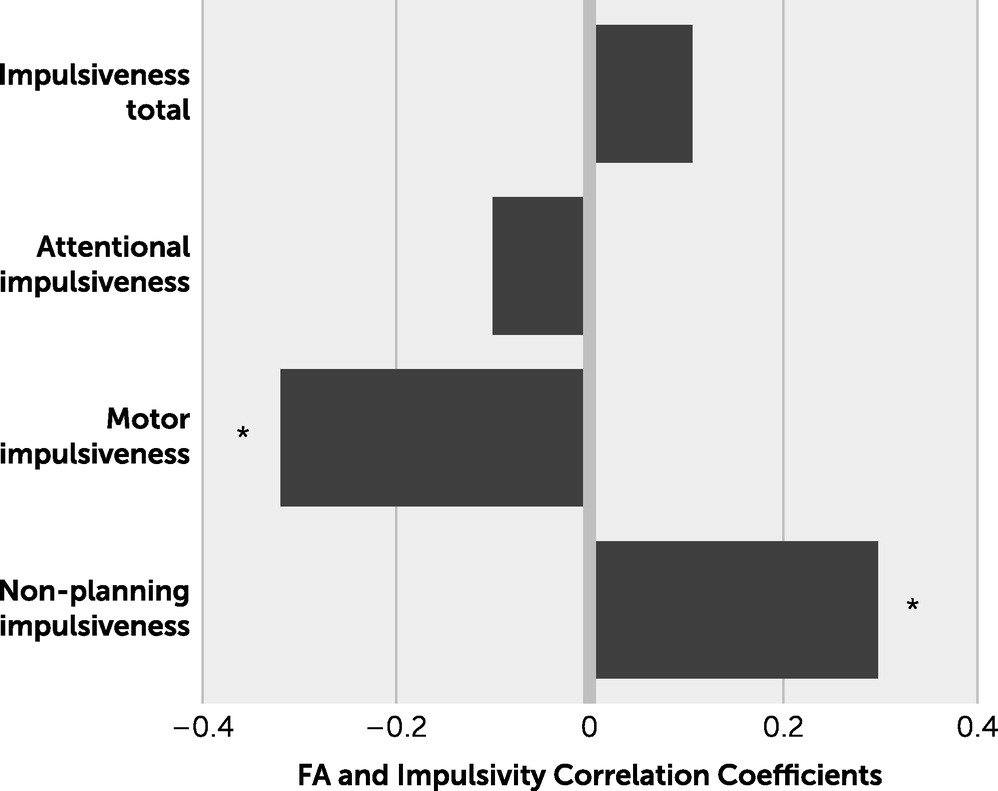
The directions of the relationship between whole-brain FA and the motor component versus the nonplanning component of impulsivity are interestingly opposite. These findings may support recent attempts to redefine the psychometrics of the BIS-11 and its factor structure. For example, in a recent report analyzing the psychometric properties of the BIS-11 using a large community sample, the BIS-11 was most consistent with a two-factor model (
29) rather than the traditional multidimensional representation. The proposed two factors, inability to wait for a reward and rapid response style, can be conceptually viewed as opposite: the former can be detrimental but the latter beneficial in everyday functions. Because motor impulsivity may be aligned with inability to wait for a reward and nonplanning impulsiveness may in part be related to rapid response style, a negative association between white matter FA for motor impulsivity and a positive association between white matter FA for nonplanning impulsivity may suggest that a poorer microstructural integrity of the whole-brain white matter is associated with motor impulsivity, but a higher integrity of the whole-brain white matter is associated with nonplanning impulsivity.
Higher FA in the white matter, including FA at the CR, anterior CR, and corpus callosum, has consistently been associated with faster information processing speed (
33,
34). Because higher levels of nonplanning impulsiveness may reflect the rapid response style described in the two-factor model for BIS-11 (
29), the finding that higher values of the CR FA are associated with higher scores on nonplanning impulsiveness may be consistent with the information processing speed function of the white matter. Moreover, nonplanning impulsivity has been found to be positively correlated with frontal gray matter volume, whereas motor and attentional subdomains were negatively correlated with superior temporal gray matter volume (
35), in parallel with the signs of our findings of an association with white matter.
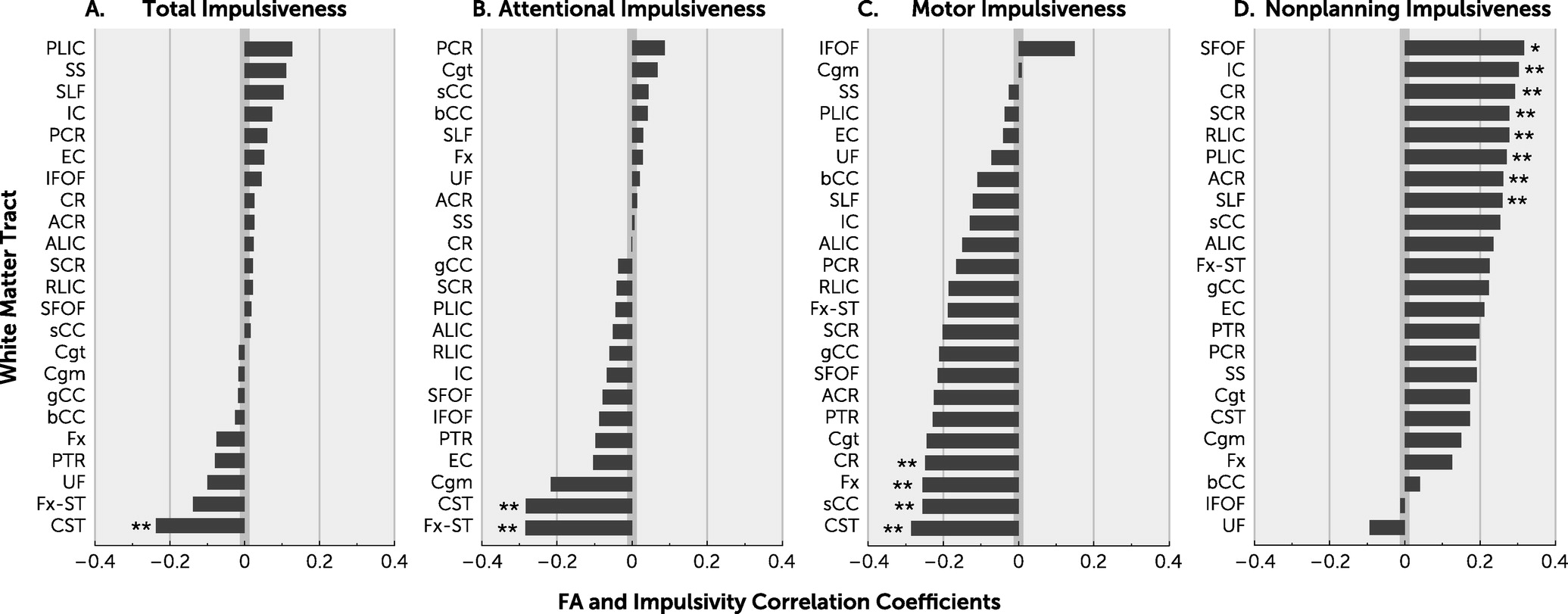
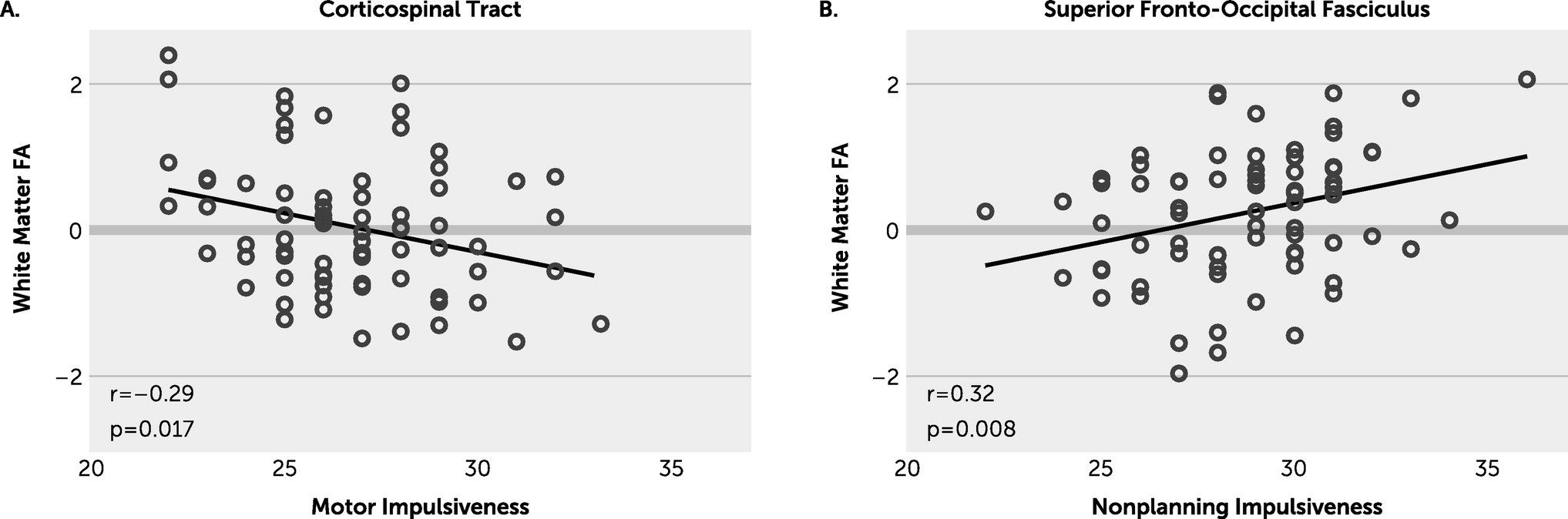
We observed an inverse association between the CST FA and motor impulsivity, suggesting that reduced FA here may contribute to more motor impulsivity. The CST underserves motoric functioning as its predominant responsibility, based on established neurobiological understandings; however, it has been shown to be more widely serving in psychological contexts as well (
36). These findings support the notion that white matter studies may assist in understanding the underlying neurobiology of complex behavioral constructs such as impulsivity.
On other specific white matter tracts, we found no significant associations with impulsivity measures that survived corrections for multiple comparisons, although this may be due to insufficient power given the need to correct for the large number of tracts and the use of healthy controls in whom white matter integrity damage and psychopathology may be more subtle than in disease states. Nevertheless, we observed nominally significant associations that appear interpretable. For example, we found an association between lower FA at the CST and higher total impulsivity score (
Figure 2A), consistent with impulsivity being largely a motor-based behavior, and impaired CST white matter microstructure may reduce impulse control output. Given that the CST was also found to trend significant in each of the other subdomains as well, these findings further strengthen our observations overall.
The white matter tract and impulsivity subdomain finding with the most association was between the SFOF and nonplanning impulsiveness. Given that nonplanning impulsiveness did reach significant correlation with whole-brain white matter FA, the SFOF is of interest. Functionally, this bundle is tasked with the role of modulating visual and spatial aspects of cognitive processing in a top-down fashion. The SFOF is responsible for processing information regarding the peripheral visual field, including aspects perceived outside of conscious cognitive processing, as discerned from lesion-based case studies, and controlling actions based on decisions with this input. Schmahmann and Pandya (
37) proposed that the connectivity between dorsolateral prefrontal and parieto-occipital areas served by this fronto-occipital fasciculus are key in the higher order aspects of motor behavior and visuospatial aspects of attention processes. Our findings of nonplanning impulsiveness mapping onto the SFOF are in stark agreement with the behavioral implications reported elsewhere.
Few robust studies of DTI and impulsivity exist. A report of 10 chronic marijuana users compared with nonmarijuana users found an association between BIS-11 score and white matter FA but only among marijuana users (
38). Another study used the Urgency, Premeditation, Perseverance, and Sensation Seeking questionnaire to measure impulsivity among 143 healthy control subjects (
15) and found that the FA of the white matter tract between prefrontal cortex and nucleus accumbens was positively correlated with impulsivity levels. The direction of the association may be counterintuitive, but these findings also support a role of white matter integrity in impulsivity that bears relevance in healthy control subjects.
Impulsivity among humans is unlikely to be controlled by isolated neuroanatomy or neurotransmitter domains. It is likely a rather complex multifactorial human behavior, influenced by multiple cortical and subcortical brain regions, in which connectivity by cerebral white matter may serve a critical role. Regional laterality may also have importance with regard to white matter microstructure and is a key difference when compared with functional neuroanatomy, in which lateralized brain regions are ubiquitously assumed to confer distinct outputs. In agreement with the largest and most robustly used DTI analytic method to date, an agnostic approach to laterality was used in the main analysis (
18,
39,
40) here as well. With accumulating data, however, lateralized microstructural integrity may similarly prove useful. We have shown here that aspects of impulsive behavior could be attributed to the integrity of the white matter microstructure connecting brain regions implicated in impulsive behaviors.
The BIS-11 is a self-reported questionnaire and, as such, is subject to user biases, including lack of insight among the participants reporting. The cross-sectional nature of the study design also limits causal interpretation between white matter integrity and impulsivity measures. Diffusion MRI parameters other than FA (such as axial, radial, and mean diffusivities) were not explored, although FA has been used as a more accurate composite means of capturing microstructural integrity. The demographic characteristics were constrained in several important dimensions, including substance use disorder over the course of the individuals’ lifetime, and subjects were only screened for more recent (6-month timeframe) habitual use of substances. Data on factors that may also contribute to white matter microstructural impairments, such as pollution and pesticide exposure, were not collected, and these should be considered in future studies. Also, white matter macrostructure data that include hyperintensities would similarly add value to future studies assessing microstructural integrity. Last, tract-specific analysis was limited by insufficient power to correct for multiple comparisons, so the tract-based interpretations should be viewed with caution; however, the findings were generally in agreement with the averaged whole-brain FA findings.