An array of persistent neuropsychiatric symptoms, such as cognitive impairment, depression, anxiety, fatigue, and insomnia, have been reported by patients in the aftermath of their initial COVID-19 infection, but there has been a call for more research to clarify the PASC syndrome (
2). Here, we share our clinical experience and observation of this new syndrome, describing the neuropsychiatric manifestations of PASC in a sample of patients seen at the Henry and Allison McCance Center for Brain Health and Neuropsychiatry Clinics at Massachusetts General Hospital. We explored whether there was any specific signature of cognitive impairment in SARS-CoV-2, and we hypothesized that clinically, PASC would have subgroup presentations and that “brain fog,” as termed by the media as well as patients, would have many different meanings, with high incidence of executive dysfunction (attentional complaints, encoding/retrieval memory problems, and word-finding difficulties), anxiety, depression, and sleep difficulties affecting frontal and subcortical networks the most. To understand this syndrome, a multidisciplinary approach is needed and extensive future research in this area is imperative and necessary in order to establish the most effective course of treatment for these patients and to facilitate robust and sustained recovery from this pandemic.
Methods
Study Design and Participants
This preliminary study represents a retrospective cohort-nested design within our prospective cohort study of COVID-19 survivors comprising men and women who have received care due to persistent COVID-19 symptoms. The majority of enrolled patients had mild illness that was managed in the outpatient setting (72.4% of those with confirmed SARS-CoV-2 infection, 92.3% of nonconfirmed cases). Patients that required hospitalization (27.6% of those with confirmed SARS-CoV-2 infection, 7.7% of nonconfirmed cases) had moderate illness at most, requiring only brief hospitalization, and no supplemental oxygen, intubation, or mechanical ventilation. Patients received care at Massachusetts General Hospital between February 2020 and May 2021. All patients were adults aged ≥18 years at the time of COVID-19 infection, except one patient whose care began a few months shy of her 17th birthday. Patients were enrolled in the multidisciplinary clinic from several weeks to several months after their acute phase of COVID-19 and thereafter met with clinicians on a regular basis. Patients without a confirmatory positive SARS-CoV-2 result by PCR or antibody diagnostic test were grouped separately as presumptive cases, and their characteristics were included in our observation. Presumptive cases were defined as those in which the patient presented with an acute illness, including fever, cough, myalgias, shortness of breath, anosmia, and/or gastrointestinal symptoms, and was living in a high endemic area for COVID-19. The study was reviewed and approved by the institutional review board at Massachusetts General Hospital.
Clinical Assessment and Ancillary Tests
The clinic accepted referrals from patients’ care team as well as self-referrals. Patients underwent a thorough neuropsychiatric assessment that included detailed history taking, general physical and neurological examinations, and “bedside” neurocognitive examination. Ancillary testing included neuroimaging, laboratory workup (not limited to complete blood count; comprehensive metabolic profile; vitamin D, vitamin B12, and folate levels; and thyroid-stimulating hormone levels), and nerve conduction studies. Patients’ care plans encompassed mental health follow-up and assessments; referrals to physical, occupational, or speech therapy; lifestyle recommendations; pharmacological sleep aids; psychotropic medications for depression and/or anxiety; and other workup/management, which may have included headache medications, pain medications, or vitamin supplements.
Data Collection
The medical records of eligible patients were reviewed retrospectively by the study staff. Retrieved data described sociodemographic characteristics, comorbidities, acute and persistent COVID-19 neuropsychiatric and non-neuropsychiatric manifestations, self-reported sleep quality, and perceived sleep-related issues. Neuropsychiatric screening, including for executive function, was reported along with other clinical assessments. If available, scores from the following psychometric and cognitive scales were included in the data collection: the Patient Health Questionnaire (PHQ)-2 (expanded to the PHQ-9 if PHQ-2 screening >3 points), the Generalized Anxiety Disorder (GAD)-2 (expanded to the GAD-7 if GAD-2 screening >3 points), the Montreal Cognitive Assessment (MoCA) test or virtual Mini-MoCA, posttraumatic stress disorder (PTSD) screen, and the Alcohol Use Disorders Identification Test–Concise (AUDIT-C). All of these screening assessments were administered in-person or via a virtual platform. In addition, neuroimaging findings among patients who underwent MRI after the acute COVID-19 episode were described. Laboratory workup and multidisciplinary management were reported. We highlighted patients’ employment status and any limitation in their capacity to return to work.
Statistical Analysis
We aimed to describe the neuropsychiatric sequelae among our patients experiencing long-lasting symptoms following initial SARS-CoV-2 infection. Estimations on symptom duration were limited to the time frame between the day of patients’ COVID-19 positive diagnostic test result and the first time they received care by a clinician in our multidisciplinary post-COVID care program. Patients’ baseline sociodemographic characteristics and clinical profiles were described. Frequencies of clinical manifestations and persistent neuropsychiatric and non-neuropsychiatric symptoms associated with COVID-19 acute illness were calculated, and the frequency of self-reported sleep quality and perceived sleep-related issues after COVID-19 infection was described. Descriptive statistics were used to quantify the results of neuropsychiatric screening tools and assessments conducted during in-person or virtual visits. In addition, laboratory workup and treatment, as well as functionality and/or ability to return to work, were recorded. Various comparison groups were established on the basis of relevant medical history or clinical characteristics affecting PASC, including previous psychiatric or neurologic disease, executive dysfunction findings, and ability to return to work. We assessed differences in sociodemographic and clinical characteristics between the study groups. Between-group differences were assessed by using chi-square test for categorical variables, independent sample t test for continuous variables with parametrical distribution, and Wilcoxon signed-rank test for continuous variables with nonparametrical distributions. A correlation matrix of the PASC neuropsychiatric manifestations was obtained. Phi coefficients were calculated per comparison pair to test how correlated or independent these manifestations were among them. Odds ratios and 95% confidence intervals for common neuropsychiatric symptoms of PASC were calculated for the main predictors of interest. The regression analysis models were adjusted for age, sex, body mass index (BMI), language, education level, and ancestry. A two-sided p value <0.05 was deemed significant. The Sidàk correction method for multiple comparisons was applied. Statistical analyses were performed by using R Studio, version 3.5.3, and Python, version 3.6.5.
Results
A total of 100 patients were included in this study. Of these, 87 had a confirmatory positive SARS-CoV-2 PCR or antibody diagnostic test result. Thirteen patients who did not have a positive confirmatory test were grouped as having presumptive SARS-CoV-2 infection. Baseline demographic and clinical characteristics of patients with confirmed infection and of those with presumptive infection are presented in
Table 1. Among patients with confirmed COVID-19 infection, 63 (72.4) were female, 22 (25.3%) were non-White, and the mean age was 49.1 years (SD=14.9). Among those grouped as having presumptive infection, 10 (76.9%) were female, four (30.8%) were non-White, and the mean age was 41.1 years (SD=16.1).
Presenting Symptomatology
Presenting PASC symptomatology spanned “brain fog,” fatigue, headache, and sleep problems. The initial presentations were similar between the confirmed and presumptive positive case groups (
Table 2). Of note, we retained “brain fog” as a term in our results because it referred to patients’ presenting complaints in their own words, often picked up from verbiage used by news outlets and referring to certain symptoms that can affect one’s ability to think, presenting as confusion, inability to focus, or difficulty expressing thoughts. Cognitive complaints in both confirmed and presumptive groups presented as memory-related (58.6% and 46.2%, respectively) or concentration/attention-related (51.7% and 46.2%) (
Table 2). Of note, 19% of patients did not report cognitive complaints. In addition, the prevalence of anxiety was estimated at 35.6% among patients with confirmed infection, followed by a prevalence of clinically significant depressive symptoms estimated at 23.0% in this same group. Other common manifestations of PASC with high prevalence were myalgia, long-lasting anosmia, and persistent dyspnea. Fewer patients presented with visual disturbances, tremors, paresthesia, dysautonomia, and small-fiber neuropathy. The average duration of symptoms among patients with confirmed infection was 176.9 days (SD=109.7). Symptoms among patients with presumptive infection ranged broadly but endured long after the acute course between 33 and 357 days (
Table 2).
Comorbidities
Among patients with confirmed infection, the most common medical comorbidity was hypertension (33.3%), followed by asthma (14.9%), cardiovascular disease (12.6%), diabetes (10.3%), and history of malignancy (8.1%). Among patients with presumptive infection, asthma and hypertension were common (30.8% and 15.4%, respectively) (
Table 1).
Among the 87 patients with confirmed infection, 50 endorsed a previous psychiatric history. Prior psychiatric diagnosis was not found to significantly influence presenting complaints in our sample; particularly, it did not affect the prevalence of depression or sleep problems.
Prior neurological history included migraine and other headache disorders, which were the most common among both confirmed (33.3%) and presumptive (76.9%) case groups (
Table 1). Prior neurological diagnosis was not found to significantly influence presenting complaints in our sample.
PTSD
Symptoms sufficient to merit a diagnosis of PTSD were reported by one (1.2%) patient in the confirmed infection subgroup. Of the 13 patients with presumptive infection, three (23.1%) endorsed prior trauma history, and four (30.8%) presented ongoing PTSD symptoms.
Sleep Disturbances
Sleep disturbances were common (74.0% of the total sample), with sleep-onset insomnia being the most frequent sleep complaint. Most participants classified their sleep quality as poor (
Table 3).
Screening “Bedside” Neuropsychiatric Scores
Neuropsychiatric assessments performed in the clinic for patients with confirmed infection, as well as for those with presumptive infection, are summarized in
Table 4. Formal PHQ-2 scores were recorded for 64/100 patients; 25 had a score >3 and therefore underwent additional screening with the PHQ-9 (
Table 4). Formal GAD-2 scores were recorded for 65/100 patients; 21 had a score >3 and therefore underwent additional screening with the GAD-7 (
Table 4). Similar prevalence of both depressive and anxious symptoms was seen for patients with and without a prior psychiatric history. Thirty patients completed the MoCA, with a mean score of 26.7 (SD=2.7) (
Table 4). Another 18 patients completed a virtual Mini-MoCA, with an average score of 11.0 (SD=1.0) (
Table 4). There were no differences in cognitive testing detected between patients with and without a prior psychiatric history.
Executive Dysfunction Symptoms
Among the 87 patients with confirmed infection, 71 (81.6%) reported symptoms of executive dysfunction, defined as the patient’s inability to plan and behavior in response to a change in the environment (
3). Patients presenting with executive dysfunction were older compared with those without symptoms of executive dysfunction (mean age=50.4 [SD=15.0] versus 44.0 [SD=13.5] years, respectively; p=0.688). There was a significantly greater prevalence of “brain fog” as the presenting subjective complaint among those with executive dysfunction (p<0.001). In addition, executive function appeared to be affected by sleep quality, with a greater percentage of poor sleep reported among those presenting with executive dysfunction compared with those without executive dysfunction symptoms (N=30 [42.3%] versus N=3 [18.8%]; p=0.143), although this result did not meet the statistical significance threshold.
Neuroradiological Findings
Of the 87 patients with confirmed SARS-CoV-2 infection and 13 patients with presumptive infection who had neuropsychiatric sequelae after their initial acute episode, 36.8% (N=32) and 38.5% (N=5) underwent brain MRI, respectively. In the confirmed group, 17 patients had no pathological findings, representing a proportion of 19.5% of the total confirmed cases and 53.1% of confirmed cases with MRI data. Findings from most to least prevalent included subcortical and/or white matter hyperintensities on T2-weighted imaging (N=9, 10.3%), vascular abnormalities (N=7, 8.1%), global volume loss (N=2, 2.3%), and altered appearance of olfactory bulbs (N=1, 1.61%). Among the patients in the presumptive group who underwent neuroimaging, three had no pathological findings, and one had subcortical and/or white matter hyperintensities. Of note, among the 32 patients with confirmed infection who underwent MRI, 25 reported symptoms of executive dysfunction. Of these 25 patients, six were found to have vascular abnormalities or infarcts, compared with one out of the seven patients who underwent MRI but did not report executive dysfunction symptoms. Patients with low clinical pretest probability of abnormal neuroimaging findings did not undergo MRI.
Follow-Up Care
A broad range of services were rendered, including referrals to physical therapy, occupational therapy, speech and language pathology, and medicine, as well as to neurological subspecialties, neuropsychological testing, mind-body medicine, and psychotherapy modalities. In the total cohort, the most common referral was to speech and language pathology for cognitive rehabilitation (31.0%), followed by neuropsychology (29.0%) and physical therapy (28.0%). Clinicians referred patients to neuropsychology to better characterize the pattern of neurocognitive deficits. Several patients were referred to a mind-body medicine program for stress management and resilience training. Others were referred to cognitive-behavioral therapy. Mental health follow-up was the most frequently recommended aspect of care (64.0%). Lifestyle recommendations, such as good sleep hygiene and self-care, were also provided to patients (27.0%).
A pharmacological sleep aid was prescribed to 30 patients (30.0%) undergoing treatment for sleep disorders (
Table 5). The main sleep aids utilized were melatonin (3–5 mg/nightly) and magnesium (500 mg/nightly), followed by gabapentin (300–600 mg/divided doses), nortriptyline (10–50 mg), amitriptyline (50–100 mg), and mirtazapine (15–30 mg). Gabapentin was used in settings with concurrent neuropathic pain. Tricyclic antidepressants were prescribed for depression and headache. Mirtazapine was used for comorbid mood disturbances. Other psychotropic interventions included use of selective serotonin reuptake inhibitors, serotonin norepinephrine inhibitors, atypical antidepressants, and beta blockers. Stimulants (methylphenidate and dextroamphetamine) were prescribed to treat impaired attention, apathy, and fatigue. Modafinil was also used in treatment of fatigue. Olanzapine or aripiprazole was continued among those already on these regimens for neuroleptic disorders. Sumatriptan was utilized for migraine treatment. Galantamine was continued for one patient with premorbid dementia.
Ability to Return to Work
Among the 77 nonretired patients with confirmed SARS-CoV-2 infection, 55 (71.4%) were able to return to work after a mean symptom duration of 186.3 days (SD=104.6), whereas 22 (28.6%) were not able to return to work after a mean symptom duration of 159.0 days (SD= 118.6), counting until the time of their last recorded appointment with their brain health provider. Differences observed between patients who were able to return to work and those who were not are summarized in
Table 6.
Correlations and Predictors of PASC Neuropsychiatric Manifestations
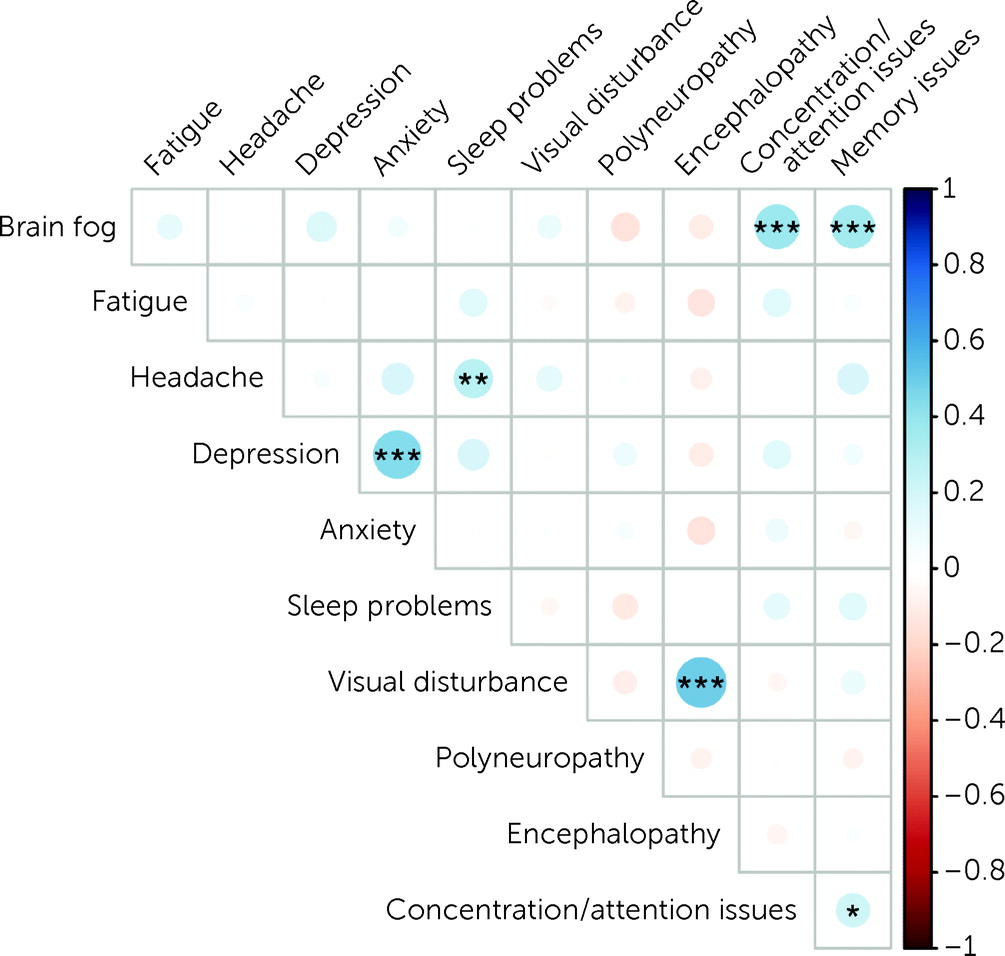
The strongest correlations were found between “brain fog” and concentration/attention issues (r=0.4; p<0.001), “brain fog” and memory issues (r=0.4; p<0.001), depression and anxiety (r=0.4; p<0.001), and encephalopathy and visual disturbances (r=0.5; p<0.001). Weaker correlations were found between headache and sleep problems (r=0.2; p=0.017) and concentration/attention issues and memory issues (r=0.2; p=0.044) (
Figure 1). Although at the start of this study, we hypothesized that premorbid conditions could affect clinical presentations, we were proven wrong. Prior diagnosis of migraine or other headache disorders appeared to be a predictor of increased odds of “brain fog” (odds ratio=5.0, 95% CI=1.6–17.6), independent of age, sex, and BMI. However, the results were no longer significant after adjusting for other sociodemographic covariates, including language, education level, and ancestry. In addition, we observed interwoven effects between prior migraine and other headache disorders and anxiety, fatigue, and depression. Similarly, depression was closely associated with complaints of “brain fog,” anxiety, and fatigue. A prior history of trauma was reported in both the confirmed infection cohort (N=16, 18.4%) and presumptive infection cohort (N=3, 23.1%), with low incidence of PASC-related acute PTSD symptoms in the confirmed and presumptive groups (N=1, 1.2%; N=0, 0.0%, respectively).
Discussion
In this observational study, we described a single-center cohort of patients experiencing persistent neuropsychiatric symptoms after confirmed or presumptive COVID-19 infection. The most common neuropsychiatric symptoms of PASC were fatigue, “brain fog,” headache, and anxiety. Other PASC manifestations included myalgia and persistent anosmia. Prior psychiatric and neurological diagnoses were frequently encountered, with anxiety, depression, and migraine being most common. The prevalence of specific PASC symptoms in patients with a history of prior psychiatric or neurologic disease fell short of statistical difference. Among patients with a confirmed diagnosis of COVID-19, the subjective symptom of “brain fog” was associated with significantly greater findings of executive dysfunction. Interestingly, these patients had a higher rate of positive neuroimaging findings compared with patients who underwent imaging without findings of executive dysfunction.
Previous studies enumerating the prevalence of cognitive dysfunction after COVID-19 infection have reported rates ranging from 20 patients out of the first 100 in the University of California, San Francisco registry (
4) to 105 patients out of 179 (58.7%) meeting criteria for moderate neurocognitive impairment in a cohort of patients from Barcelona, Spain (
5). Patients can show signs of encephalopathy secondary to COVID-19, presenting as a generalized cognitive disturbance (
6). However, studies are emerging in the literature showing that impairments in executive functioning, such as working memory, set-shifting, attention, and processing speed, are identified among COVID-19 patients (
7), similar to that described in our cohort. Previous investigators have reported a correlation between fatigue and executive dysfunction among COVID-19 patients (
8), which they propose may be due to dysfunction in GABAergic networks (
9). In line with our study, dysexecutive symptoms have also been shown in previous work to be associated with higher scores on screening surveys for anxiety and depression (
10), highlighting the important role mood plays in overall cognition. The higher burden of vascular abnormalities observed in MRIs of our patients with executive dysfunction is interesting but should be interpreted with caution given the small sample size and lack of a control population. Vascular abnormalities, such as leukoaraiosis and chronic microvascular ischemia, are common in the general adult population; if overrepresented in our patient cohort, these abnormalities suggest that preexisting structural disease may predispose to executive dysfunction or other neurologic sequelae. Some studies have linked the higher burden of subcortical vascular abnormalities in individuals with dysexecutive syndrome to an apathy syndrome. It has been suggested that SARS-CoV-2 targets the frontal lobe, explaining extreme fatigue, abulia, and, in some cases, postviral mutism (
11). Previous neuroimaging studies have also described more acute vascular and ischemic findings in populations of COVID-19 survivors (
12) and nonsurvivors (
13,
14). Ultimately, more research is needed to characterize the association between PASC and cerebrovascular disease and injury. However, given that most patients with PASC do not have significant hypoxia, nor do many reflect the typical cerebrovascular risk factor demographic profile, it seems unlikely that this represents a dominant mechanistic pathway (
1,
15).
In our examination of patients with PASC over a 15-month period (February 2020–May 2021), we observed four different subgroups. First, one group of patients had predominant executive dysfunction (frontal-subcortical profile), presenting with attentional and naming difficulties, as well as confusion (“brain fog”), and—to the extreme—mutism, apathy, and severe fatigue. A second subgroup of patients presented with amnestic disorder, but upon examination, memory issues were also found to be related to frontal-subcortical circuitry dysfunction, with encoding and retrieval as the main issues. Third, in some patients, PASC worsened preexisting disorders, such as migraine and other headache disorders, depression, and anxiety, as well as sleep disorders. In a fourth group of patients, PASC may have unmasked an unknown underlying disorder (e.g., neurodegenerative disorders, seizures, hypertension, and cardiac disease).
While the pathology and mechanisms underlying the four observed PASC subgroups and the persistent neurocognitive symptoms associated with COVID-19 remain incompletely understood, recent promising research has highlighted the potential role of immune-mediated injury. In particular, given the substantial clinical overlap with certain other chronic neuropsychiatric disorders (
16–
19) (i.e., chronic fatigue syndrome, fibromyalgia, postconcussive disorder, immune effector cell-associated neurotoxicity syndrome, and other postinfectious syndromes), substantial effort has been placed at attempting to identify potentially unifying pathways. In a study of cancer patients with neurologic manifestations of COVID-19, several proinflammatory cytokines were found to be enriched in the CSF of patients with persistent neurologic symptoms 2 months after their initial COVID-19 diagnosis, suggesting a potentially immune-mediated injury (
19). A number of nonspecific histopathological abnormalities have been identified from autopsy series of COVID-19 nonsurvivors; although some mild degree of perivascular and parenchymal inflammation is occasionally observed, there is no widespread or highly prevalent inflammatory process to suggest an obligatory alloimmune reaction (
20). One recent autopsy study noted the presence of megakaryocytes in brain capillaries, suggesting that this atypical finding could be evidence of widespread endotheliitis (
21). Central nervous system (CNS) endothelial dysfunction with resultant blood-brain barrier disruption has been proposed as a pauci-cellular mechanism that may underly other incompletely understood mechanisms characterized by presumed autoimmune neurologic syndromes (
22). Of note is a recent nuclear transcriptome study that profiled 65,309 transcriptomes from 30 frontal cortical and choroid plexus samples from eight patients with severe COVID-19 and compared them with samples from 14 control subjects (
23). The investigators found no SARS-CoV-2 in the CNS samples but did note cellular changes, suggesting that choroid plexus barrier cells relay peripheral inflammatory signals into the brain along with infiltrating peripheral T cells. In addition, CNS microglia and astrocytes showed signs of activation by transmitted peripheral signals.
These glial cells may be particularly vulnerable to circulating inflammatory mediators when they reside close to circumventricular organs that are relatively devoid of blood-brain barrier protection (
24). The organum vasculosum of the lamina terminalis may be an important candidate zone given its proximity to the anterior cingulate cortex, an area that, when impaired, can lead to abulia apathy syndrome (
25). These findings are significant because they suggest that earlier treatment with medications to minimize endothelial activation (i.e., anti-inflammatory medications such as dexamethasone), even in cases with relatively mild acute respiratory symptomology, may stave off the subsequent development of persistent neurocognitive symptoms. This is an area warranting additional prospective investigation.
Moreover, the neuroinflammatory response does seem to be implicated only in vascular findings. Currently, there is epidemiologic evidence suggesting a connection between PASC and PTSD and a growing appreciation of the inflammatory response syndrome that often accompanies PTSD, and this may emerge as a risk factor for PASC (
26). Given the expected psychological trauma associated with enduring the COVID-19 pandemic, it is likely that our figures underestimate the true incidence of PTSD after COVID-19, potentially due to underreporting or confounding of the associated symptoms. Longitudinal studies of the current estimates and changes to the incidences and characteristics of new diagnoses of PTSD should be undertaken to understand how the shared trauma of the global pandemic may manifest in the presentation of PASC and/or PTSD.
Finally, the ability to return to work is a crucial component of quality of life and one of the most salient metrics of outcome from a public health and economic perspective. In our study, 100% of patients that had not returned to work were female, and the majority were patients with a prior history of mental illness. Those who required hospitalization were also disproportionally affected in their inability to return to work. Although gender, ancestry, and mental illness have been rightfully at the forefront of the public conversation regarding disparities in the context of the COVID-19 pandemic (
27,
28), we need to do more to understand the factors highlighting return-to-work guidelines that encompass disparities, particularly considering that some groups classified as essential workers remained on the job despite the outbreaks in their communities or due to their economic situation (
29). An additional observation of the present cohort is that the majority of the patients seeking care at the brain health clinics were predominantly White non-Hispanic patients, precisely the population that has been shown to be less likely to experience poor outcomes from COVID-19 (
30,
31). This finding raises important questions about PASC as a disease among groups who have the opportunity to seek care, access care, and recover. Of note, our sample size was small, and our hospital catchment area is predominantly White, which could have played a role in these findings. A multidisciplinary, multilevel approach is clearly needed to tackle the health implications among those placed at increased risk of COVID-19 infection and postviral sequelae, along with decreased access to adequate health care and paid sick leave. The implementation of equitable policies is needed to address these immense gaps in equity.
Limitations
The small sample size of this single-center study limits both the generalizability of our findings as well as our ability to find statistically significant differences within subpopulations of the cohort. Furthermore, as this was a retrospective review of clinical encounters while virtual visits were being troubleshooted and expanded, many encounters were missing aspects of psychometric data, such as a full PHQ-9 or a complete MoCA. This further limited our ability to derive definitive conclusions regarding symptom burden and cognitive impairment. Also, the lack of a control group limited our ability to attribute our findings specifically to COVID-19 infection. In addition, because COVID-19 is a novel coronavirus, even our lengthiest patient follow-up was only about 1 year, making it hard to designate long-term outcomes. Finally, selection biases remain an issue, not necessarily mitigated by sample size.
In summary, these preliminary findings suggest that PASC varies in frequency and duration according to premorbid history and affects functional domains and the ability to return to work. We have seen premorbid diseases worsen and others get unmasked. Longitudinal research with larger cohorts is needed to characterize PASC and the related neuroinflammatory response in vascular disease and PTSD, as well as to quantify the effect of premorbid conditions. Understanding the influence of culture and ethnicity on the pandemic experience and symptom reporting is essential in providing optimal care and implementing public health measures.