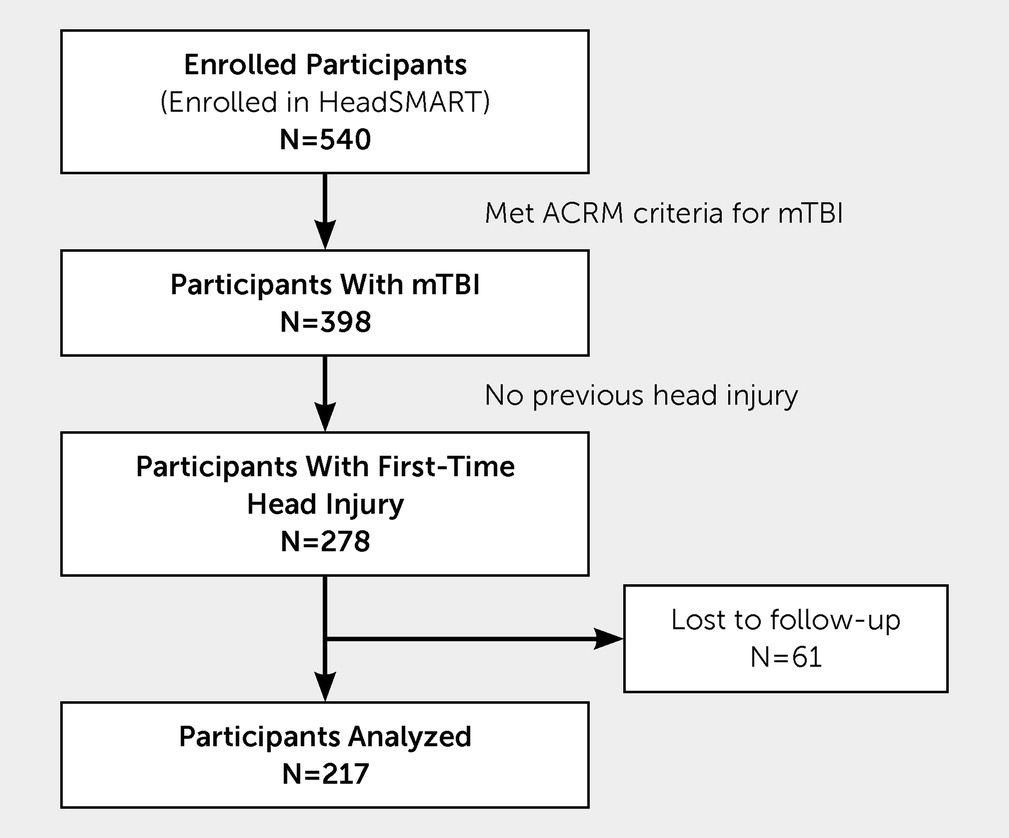
Traumatic brain injury (TBI) is a major public health problem, with more than 2.5 million people seeking medical care annually, of which 70%−90% experience mild TBI (mTBI) (
1–
6). It is further estimated that 5.3 million Americans are living with a TBI-related disability, costing the United States an estimated $75.6 billion in annual direct and indirect medical costs (
7). mTBI constitutes a heterogeneous population for a variety of reasons, including a great variance in persistence and severity of symptoms postinjury. Patients with mTBI are found to have higher rates of neuropsychiatric symptoms, postconcussive symptoms (e.g., headaches, blurred vision, body pain, dizziness, and light sensitivity), and poorer global outcomes than those with more severe TBI (
8–
11). The presence of these symptoms can detrimentally affect recovery and quality of life postinjury (
12–
16).
Depressive symptoms are among the most common neuropsychiatric sequelae of mTBI and occur in 17%−31% of persons with such injuries (
11,
17–
27). Depressive symptoms include feelings of sadness, loss of interest, lethargy, amotivation, sleep disturbance, appetite changes, irritability, and suicidal thoughts. Depressive symptoms after TBI (regardless of TBI severity) increase suicide risk; interfere with engagement in activities of daily living; and impair rehabilitative, social, occupational, and interpersonal functioning, thereby adding to chronic TBI-related disability and increased global burden of disease (
28–
31).
Well-established demographic and clinical risk factors for depression after TBI include being older, being female, having less than a high school education, poor pre-TBI psychosocial functioning, higher TBI severity, substance use, comorbid sleep and cognitive disturbances, frontal lesions, and pre-TBI psychiatric illness (
22,
23,
32–
40). Although these studies have focused on cohorts of heterogeneous TBI severities (
30,
40–
47), correlates of developing depression after first-time mTBI have not been an area of research focus. Despite the growing literature on understanding neuropsychiatric consequences of mTBI (
41), cohort studies have not focused on depression as a primary outcome within the first year postinjury and have primarily examined other measures, such as poor functional recovery and postconcussive symptoms (
48–
51). To understand why depressive symptoms might follow a single, first-time mTBI, it is useful to compare those who develop new-onset depressive symptoms after a single injury with those who do not and also with those with a preinjury depression history. By only including individuals for whom this is their first TBI (of any severity), any cumulative effect of multiple brain injuries can be ruled out as a contributor of persistent symptoms. Understanding correlates of first-time depressive symptoms that develop in the early TBI period (i.e., within the first 6 months) can help identify who is at risk and then apply interventions to prevent the subsequent progression of disease.
To date, only one study has examined the risk factors, prevalence, and correlates for new-onset depression specifically in mTBI (
52). Rao et al. (
52) longitudinally followed 43 participants for 1 year and found the prevalence of new-onset depression to be 18%. Older age and presence of frontal subdural hemorrhage were the only two significant findings noted in the depressed group compared with the nondepressed group. Our study aims to build on this knowledge through characterizing a cohort with first-time mTBI by analyzing demographic and clinical variables of those who develop depressive symptoms within the first 6 months postinjury as compared with those who do not. Given the findings in previous studies of individuals after TBIs (of all severities) who develop depression, we investigate whether individuals with first-time mTBI who develop depressive symptoms are more likely to be female, be older, have less education, have brain lesions, and experience worse postconcussive symptoms and incomplete functional recovery within the first 6 months postinjury as compared with individuals with first-time mTBI who are not depressed. Additionally, the prevalence of new-onset depressive symptoms versus recurrent depressive symptoms (those with a preinjury history of depression) will be established. It will be determined whether individuals who develop new-onset depressive symptoms differ from those who develop recurrent depressive symptoms as they relate to postconcussive symptoms and functional recovery and whether these two groups differ from those who do not develop any depressive symptoms after TBI.
Discussion
This study is one of the first, to our knowledge, to examine the prevalence and correlates of depressive symptoms within the first 6 months of first-time mTBI in a prospective cohort. Our findings revealed that individuals with depressive symptoms postinjury were more likely to be female and non-White (consistent with the broader TBI literature); those with depressive symptoms had higher postconcussive symptoms at 3 and 6 months compared with those with no depressive symptoms (consistent with the literature); and those with depressive symptoms were less likely to have complete functional recovery at 3 and 6 months, compared with those with no depressive symptoms; however, there was no statistically significant difference in postconcussive symptoms and functional recovery between those with new-onset depressive symptoms and those with recurrent depressive symptoms. The percentage of individuals with brain lesions did not differ between those who developed depressive symptoms and those who did not postinjury. Of note, these study findings reveal that there exists a subset of individuals (12% of our cohort) who developed clinically significant new-onset depressive symptoms after their first mTBI, which is consistent with the findings of a previous study examining depression after mTBI in a smaller cohort (
52). These individuals did not differ demographically from those who had recurrent depressive symptoms after first-time mTBI (11% of the cohort). Individuals within both groups who developed clinically significant depressive symptoms were more likely to be women and persons of color than individuals who did not have significant depressive symptoms after injury. Individuals with depressive symptoms, whether new onset or recurrent, after mTBI are at higher risk for clinically significant postconcussive symptoms and incomplete functional recovery for the first 6 months postinjury.
The findings in this study of being female, being older, and not being White as correlates of the development of clinically significant depressive symptoms after mTBI are consistent with what has been established in the broader literature for TBI of all severities of injuries (majority moderate and severe TBI) (
33–
35,
38). Further, the findings that depressive symptoms after mTBI are associated with persistent postconcussive symptoms and higher risk for poor functional recovery in the first 6 months has also been reported in the TBI literature (
65,
66). It has been postulated that women’s depressive symptoms during the early recovery period in mTBI and moderate TBI might be explained by higher symptom load and perceived stress, yet mechanisms responsible for these differences have not been explored (
18,
22,
24). It has been shown that older patients have a higher risk of functional decline with a greater degree of disability 5 years postinjury compared with younger patients. This has been thought to be due to an increased number of actual and perceived health problems for older patients as compared with younger patients (
67). Reasons why individuals who are not White are at higher risk for incomplete functional recovery have not been explored.
The findings of this study suggest that depressive symptoms may be associated with greater persistent postconcussive symptoms beyond 3 months and incomplete functional recovery in the early mTBI period. Those with new-onset depressive symptoms after first-time mTBI are at similar odds of incomplete functional recovery as those with recurrent depressive symptoms, suggesting that those with a preinjury diagnosis of depression are not the only patients at risk for impaired recovery in the chronic mTBI period. This study highlights the importance of monitoring for the presence of depressive symptoms across the first 6 months postinjury. It is important to note that the majority of those with mTBI recover from postconcussive symptoms within 7–10 days after injury; however, 10%−15% persist with chronic symptoms after 3 months (
67,
68). In this minority of cases, functional impairment can exist in up to 53% of individuals at 1 year postinjury, as recently described in a report from the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) research group (
69). Impaired functional recovery may be affected by not only persistent postconcussive symptoms but also the presence of clinically significant mood and vegetative changes, emphasizing the importance of immediate and frequent monitoring of depressive symptoms in the early period after mTBI.
Although our study reports on a modest sample size when compared with larger multisite studies that examine civilian populations, the findings remain consistent with demographic and preinjury correlates that were found in these larger studies. For example, in the aforementioned TRACK-TBI study, Stein et al. (
41) found that of 1,155 participants with mTBI, those with lower education, who were Black, and those who had a history of mental health problems were at substantially increased risk of depression. The Early Predictors of Outcome after Mild Traumatic Brain Injury (UPFRONT) study, which followed 910 participants with mTBI within the first 6 months postinjury, found that emotional distress and maladaptive coping experienced early after injury, in combination with preinjury mental health problems, education, and age, were important predictors for recovery at 6 months after mTBI (
48). What is important to note is that, similar to our study, these larger studies also followed participants in the early TBI period (within the first 6 months of injury), reflecting the growing interest within the field to better understand symptom trajectories and predictors of outcomes immediately after mTBI. These larger studies did not however, examine the onset patterns of depressive symptoms (new onset versus recurrent) in the manner done in the present study.
Our study also underscores the importance of ensuring that clinicians are monitoring the concomitant trajectory of postconcussive and depressive symptoms in conjunction with functional status at the time of injury into and beyond the chronic injury phase (i.e., after 3 months postinjury). Proactive interventions early in the mTBI recovery period may help to prevent, or at least mitigate, the development of depressive symptoms. These may include psychoeducation, psychotherapy, psychosocial rehabilitation, neurorehabilitation, and psychotropic medication treatments to prevent the emergence of symptoms and associated disability.
It is important to note the limitations of our study. First, the PHQ-9 scale was used to assess depressive symptoms, but formal psychiatric assessments such as the SCID-5 to diagnose major depressive disorder were not completed (
70,
71). Although in-person and telephone evaluations with semistructured psychiatric interviews were conducted by a neuropsychiatrist (M.P., D.R., or V.R.), psychiatric disorders were not formally assessed. As such, a true diagnosis of depression after mTBI could not be made, and we relied on depressive symptoms as a surrogate.
Second, this study only analyzed data from those with first-time mTBI; thus, the findings do not necessarily generalize to individuals who have a history of multiple mTBIs. Individuals with multiple TBIs are documented to have higher rates of neuropsychiatric conditions; therefore, it is likely that those with this type of mTBI history may have scored higher on the PHQ-9 and Rivermead scales (
72–
74).
Third, there is overlap in symptomatology between domains on the PHQ-9 and Rivermead scales (e.g., sleep disturbance, poor concentration, fatigue, and feeling depressed) (
60,
61,
64). Although both scales have been validated for use with TBI symptoms, it is possible that some symptoms endorsed on the PHQ-9 were, in fact, reflective of the presence of postconcussive symptoms (e.g., sleep disturbance and feeling depressed) to some degree. We conducted post hoc analyses to address the potential construct overlap between the PHQ-9 and Rivermead scales with regard to depressive symptoms. In these post hoc analyses in which the overlapping symptoms were removed from the Rivermead questionnaire, the findings remained the same. Specifically, when comparing the depressive symptoms group to the no depressive symptoms group on postconcussive symptoms, a simple linear regression was calculated at each follow-up time point (i.e., 3 months and 6 months postinjury). There was a statistically significant difference in postconcussive symptoms (p<0.001; i.e., fewer postconcussive symptoms in the no depressive symptoms group) at 3 months and 6 months postinjury. The depressive symptoms group had a 13.8 higher score than the no depressive symptoms group at 3 months postinjury and a 15.5-point higher score at 6 months postinjury. These findings suggest that the relationship between the presence of depressive symptoms and postconcussive symptoms at follow-up time points is not due to overlap between the two constructs.
Fourth, the analyses did not consider body polytrauma or extracranial injuries as a factor that could result in a difficulty in performing self-care and daily activities and in returning to work, which are all factors that might exacerbate depressive symptoms. An analysis of body injury, however, was conducted previously on this cohort, focusing on the prevalence of incomplete functional and symptomatic recovery among patients with “head injury but brain injury debatable” (HIBRID) (
75). Compared with trauma and healthy control subjects, the HIBRID group had a higher incidence of moderate/severe depressive symptoms and a similar incidence of moderate/severe postconcussive symptoms (
53).
Fifth, the presence of alcohol and substance use was not included in this analysis. It is an important factor in the persistence of symptoms in previous studies and should be included as a factor in future studies (
75,
76).
Sixth, it should be noted that those with past depression history but no depressive symptoms postinjury may have been receiving either pharmacologic or psychotherapeutic treatment. Although a pre-TBI history of depression would not be protective, being on such treatment would potentially mitigate the persistence of these symptoms after mTBI.
Despite these limitations, the primary strengths of this study are that it provides one of the first analyses of the development of depressive symptoms in a cohort of individuals with first-time mTBI, as well as the correlates of developing depressive symptoms. Being female and being non-White are associated with the development of depressive symptoms within the first 3 months after mTBI, and those with depressive symptoms are at higher risk for postconcussive symptoms and incomplete functional recovery within the first 6 months after injury. There is a rather notable cohort of individuals (12%) who develop new-onset depressive symptoms after mTBI. Taken together, these findings add to the existing literature by identifying correlates for the development of depressive symptoms and poor outcomes after mTBI, thus providing further evidence that “mild” injuries can result in clinically significant symptoms and functional decline that warrant clinical attention.