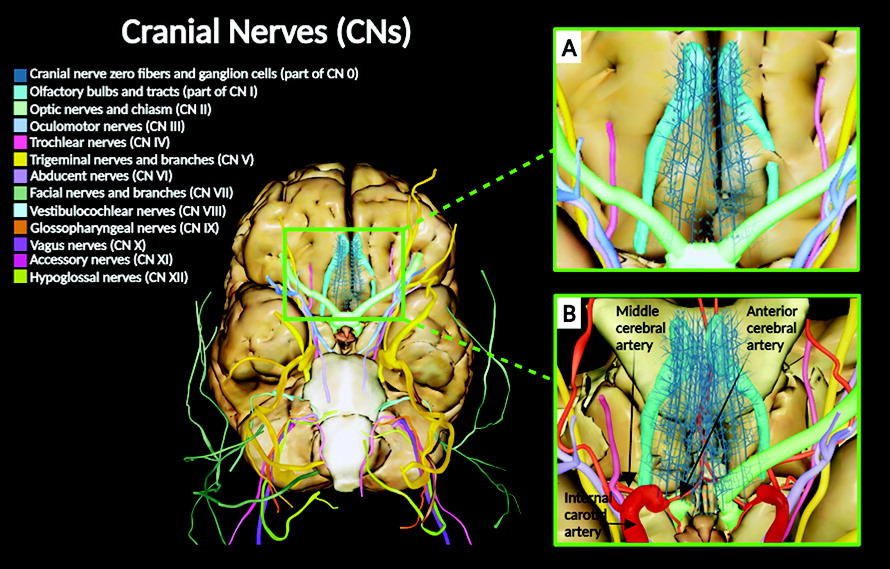
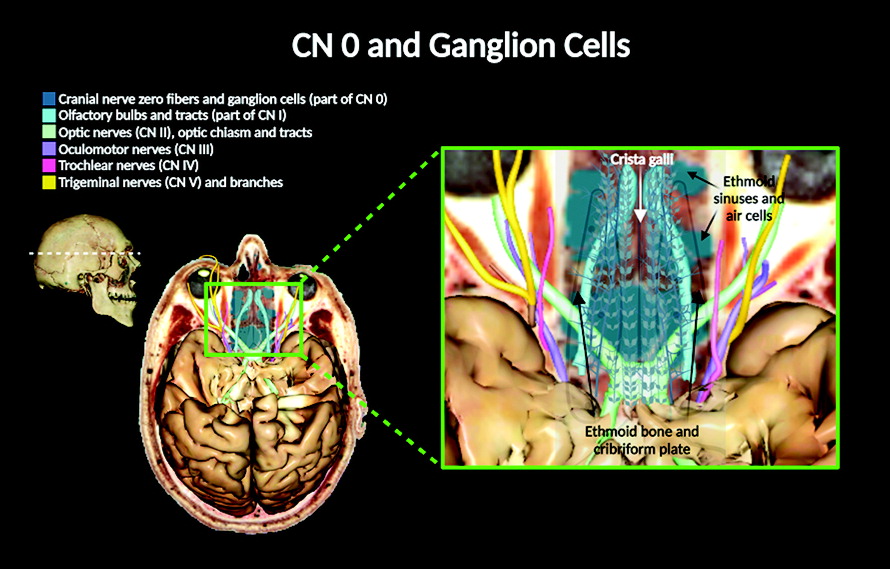
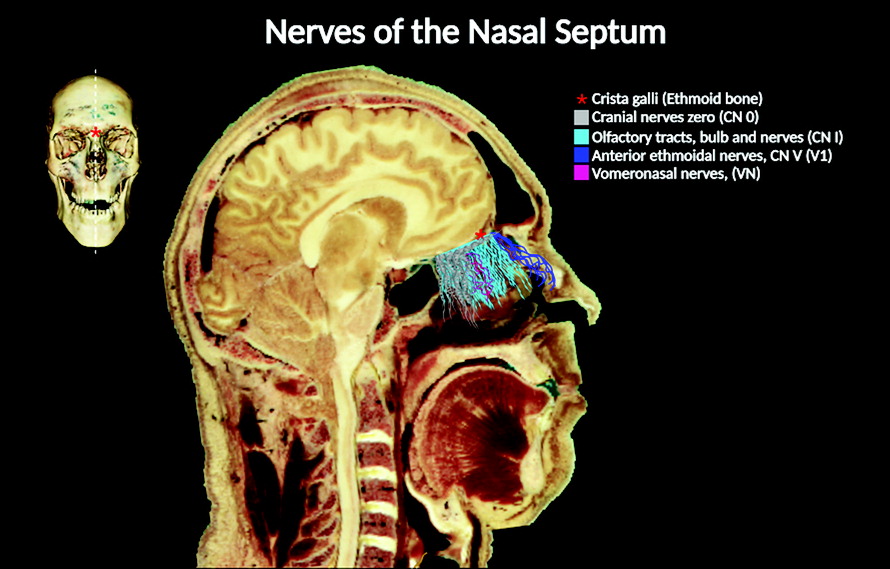
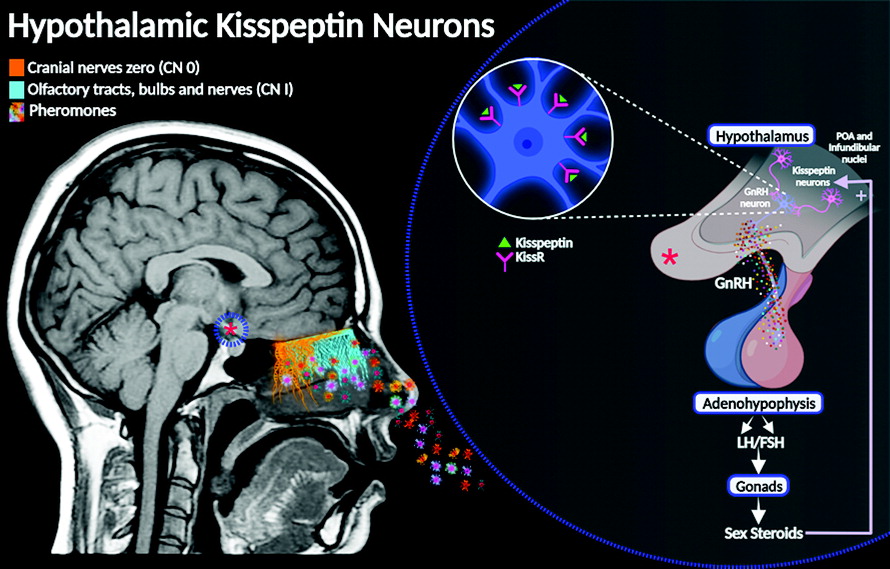
Anatomical descriptions of the cranial nerves (CNs) in humans have been historically documented in numerous scientific reports, starting from the classical Greek manuscripts to today’s contemporary medicine (
15). Our understanding of the anatomy of human CNs, including their functional components, progressed from 1500 to 1900 C.E. through cadaveric dissections. These dissections were followed by detailed anatomical and scientific reports conducted by anatomists and surgeons of that era (
16). The historical records emerging from well-known anatomists, from Galen to von Sömmerring, led to the canonical classification of the 12 pairs of CNs acknowledged in most medical textbooks and the anatomical literature (
15–
17). The order and the anatomical descriptions assigned to each CN were inspired by each CN’s anatomical positioning (i.e., the relationship to other structures), functional relevance, and, in rare cases, appearance (
15,
16,
18). Anatomically, CNs are peripheral nerves that exit the cranium or enter the cranium. They are designated by a Roman numeral (i.e., I–XII), as well as a functional, locative, or other nonnumeric name (e.g., olfactory: CN I; optic: CN II; oculomotor: CN III; trochlear: CN IV; trigeminal: CN V; abducens: VI; facial: CN VII; vestibulocochlear: VIII; glossopharyngeal: IX; vagus: X; accessory: XI; and hypoglossal: XII).
Interestingly, very little is known regarding cranial nerve zero (CN 0). CN 0 was initially known by other names, such as
nervus terminalis, nerve of Pinkus,
tractus olfacto-commissuralis, new nerve, terminal nerve,
nerve nulla (i.e., nothing, zero), and cranial nerve 13 (
1,
4,
9,
19). This enigmatic nerve was initially discovered in elasmobranchs (a subclass of Chondrichthyes, or cartilaginous fish) in 1878 by Fritsch, who described it using the term “supernumerary nerve.” At the time, the classification of CNs was already established by using Roman numeral symbols as noted above (
1). This nerve has been described in a variety of species, including humans in 1905, and it is now usually referred to as CN 0 (
1). This name is consistent with the nerve’s rostral display relative to the other CNs and thereby conforms to the preestablished numeric order of human CNs (
19). Furthermore, CN 0 was the name officially designated in the
Terminología Anatómica (issued in 1998 by the Federative Committee on Anatomical Terminology of the International Federation of Associations of Anatomists) (
20,
21). CN 0 has been distinctly described in human brain of fetuses, infants, and adults (
19). Subsequent studies have provided insights into its embryology, histological structure, neurophysiology, and clinical significance (
9).
Embryology
CN 0 is one of the most enigmatic nervous system structures among vertebrates. Similar to other CNs, its embryological origins rest in synergistic developmental interactions between the neural crest and sensory placodes (
22,
23). The neural crest is an important embryological structure comprising multipotent cells that differentiate into several cell types and contribute to many nervous system components (e.g., CNs, peripheral nerves, ganglia) and craniofacial tissues (e.g., cartilage and bones). Placodes are nervous tissue precursors that potentiate the development of sensory structures, such as the neurons of the nose that form the olfactory epithelium, the inner hair cells, and related tissues (
24). The formation of CN 0 occurs at the frontmost boundary of the migrating neural crest cells, the termination of the neural tube, and at the adenohypophyseal and olfactory placodes (
22). The embryological origin of CN 0 is similar to that of the rest of the human olfactory nerve structures, such as the olfactory nerves (CN I), the olfactory bulbs, and the vomeronasal organ (VNO) (
1,
7,
25). It comprises one to two nerve bundles passing through the anterior end of the cribriform plate (
7). Evidence suggests that these nerve fibers enter the brain along with the olfactory nerves and those of the vomeronasal processes at embryological stages 17 and 18 (i.e., Carnegie stages 17 and 18 of human embryonic development) (
7,
26,
27). A recent embryological study employing classical histological techniques reported the identification of the VNO and CN 0 fibers in parasagittal sections of 7-week-old (19-mm) human embryos (
1).
A unique and interesting feature of this nerve is its structural organization. In most species (including humans), the nerve is composed of axons expressing immunoreactivity to the decapeptide gonadotropin-releasing hormone (GnRH) (
1,
4,
22). The GnRH neuroendocrine cells and the nerve fibers of CN 0 mainly arise from the olfactory placode with neural crest contributions (
22,
28). However, the literature suggests that GnRH neurons may also arise from other embryological origins (
23,
29–
31). GnRH neurons of the hypothalamus differentiate and develop from outside the diencephalon into the forebrain, following a migratory route out of the placodal epithelium that carries the central fibers of CN 0, combined with CN I and the VNO (
32). Faulty embryological processes (i.e., inadequate migration and genetic mutations) may result in reproductive issues and other physiological disturbances, including anosmia in some cases. Currently, some of the factors modulating GnRH neuronal migration are known, but the exact mechanisms regulating the migratory processes remain inconclusive (
33).
Structure and Function
CN 0 is identifiable in human brain across neurodevelopmental stages, including in embryos, fetuses, children, and adults (
2,
34–
37). A recent study of human fetuses identified ganglion cell bodies along the crista galli of the ethmoid bone at 10 weeks (crown-rump length [CRL]: 76 mm) (
1). Another study (30 fetuses at 7–18 weeks; CRL: 25–160 mm) reported its location at the anterior edge of the perpendicular lamina of the ethmoid bone. The bundles of fibers were observed running along and crossing posterior to the nasal branch of the anterior ethmoidal nerve (a branch of the ophthalmic division of CN V). Groups of small ganglionic cells by the crista galli, whose axons constitute most of CN 0, were also observed (
7). In adults, the nerve keeps its plexiform structure and has ganglionic cells appearing in the ethmoid bone within the region of the cribriform plate. The nerve fibers appear to travel by the olfactory trigone close to the medial olfactory gyrus and lamina terminalis of the midbrain (
1,
2) (
Figure 1).
The location and distribution of CN 0 within the nasal cavity and the cranium are distinct. In the nasal cavity, its fibers intermingle with those of the vomeronasal and olfactory nerves, respectively. However, CN 0 fibers travel medially compared with CN I fibers (
1). CN 0 fibers can be distinguished from those of CN I and vomeronasal nerves on the basis of their anatomic characteristics. Whereas fibers of the CN 0 travel in the superior and anterior portions of the nasal cavity branching from its ganglionic cells, CN I fibers travel toward the olfactory bulb. In addition, the vomeronasal nerve fibers arise from the VNO (
1,
7,
35,
36).
Intracranially, CN 0 fibers appear by the olfactory bulbs (over the dura mater) or embedded within the dura mater of the brain (
37). CN 0 and the vomeronasal nerves penetrate and travel through the cribriform plate, where the plexiform fibers of CN 0 can be discerned along its ganglionic cell bodies over the cribriform plate surface (
2,
6) (
Figures 2, 3). Evidence suggests that these ganglionic cells appear as early as 6 weeks in human development (
26). Ventrally on the brain surface and lateral to the olfactory bulbs, CN 0 and the vomeronasal nerves rejoin course along the CN I fibers. The vomeronasal and CN I fibers travel to the olfactory bulbs, where second-order neurons synapse (at the glomeruli), projecting to the vomeronasal and olfactory brain cortices, respectively (
3). The CN 0 plexus travels parallel to the olfactory tracts in proximity to the septal region, close to the bifurcation of the anterior and middle cerebral arteries (
Figure 1B). Its fibers are unmyelinated and travel next to the dura mater, passing into the subarachnoid space and finally connecting to the pia mater at the gyri recti of the frontal lobes (
2,
37,
38). Some investigators contend that the precise anatomical location where CN 0 enters the brain is ambiguous (
1). However, one of its initial monikers is the terminal nerve or nervus terminalis, implying that it enters the forebrain via the lamina terminalis (
19).
Despite the anatomical relationship of CN 0 to the vomeronasal nerve and CN I fibers, its function is distinct from that of both of these other structures. Given its physiological correlation with GnRH expression, CN 0 is functionally associated with the neuroendocrine regulation of reproductive behavior, among other functions (e.g., olfaction, autonomic and vasomotor regulation, paracrine secretion of nitric oxide, and immunologic defense mechanisms) (
4,
25,
39). The axons of CN 0 support the migration of GnRH neurons to the hypothalamus, thereby potentiating the development of the hypothalamic-pituitary-gonadal axis in varied species, including humans (
8,
23,
40,
41). In addition, the GnRH component of CN 0 has been suggested to be neuromodulatory, exerting a level of neurophysiological regulation over the olfactory epithelium, thus making pheromones more readily detectable (
8).
The neuromodulatory role of CN 0 in reproductive behavior via GnRH poses a thought-provoking linkage with the hypothalamic “kisspeptin neuronal network” (KP) (
9). Kisspeptin is a neuropeptide, encoded by
KISS1, originally identified as a metastasis suppressor gene (
42,
43). It binds to the kisspeptin receptor (KissR), which was first recognized as an orphan G-protein-coupled receptor-54 (
44). The KP neural circuit is involved in the central endocrinological regulation of sexual development and human reproductive functions, essentially by inducing GnRH secretion from the hypothalamus. Physiologically, this network regulates the response of the gonadotropins, follicle-stimulating hormone, and luteinizing hormone, inducing the synthesis and release of the gonadal steroid hormones testosterone and estradiol. These hormones are essential for the physiology of reproductive behavior (
12–
14). Researchers hypothesize that CN 0 may trigger hormonal responses independently or together with other neurophysiological pathways of the brain, such as the KP neural circuit (
1,
9) (
Figure 4).
Clinical Implications
The normal development of the GnRH neural circuit is imperative for functional reproduction in vertebrates. In humans, any disturbances in the development of the nasal placode and/or migration of GnRH neurocircuitry may result in various forms of hypogonadism, including Kallmann syndrome. Kallmann syndrome is a rare genetic condition characterized by hypogonadotropic hypogonadism, typically causing lack of sexual development and anosmia (
45,
46). Clinical studies have revealed that loss-of-function mutations in the kisspeptin 1 gene (
KISS1) result in hypogonadotropic hypogonadism or precocious puberty (based on the type of mutation), indicating that kisspeptin plays an essential role in normal gonadotropin-releasing hormone physiology and in puberty (
42,
47,
48).
In the central nervous system (CNS), the
KISS1 gene is primarily expressed in the hypothalamus, functioning as an essential gatekeeper of the GnRH reproductive circuit, allowing the integration of central and peripheral inputs (
42,
49). However, the
KISS1 gene is expressed in other locations within the CNS; therefore, kisspeptin signaling is not restricted to the hypothalamic region (
50). In humans,
KISS1 has been identified in the amygdala, caudate, cingulate, globus pallidus, hippocampus, medial and superior frontal gyri, nucleus accumbens, parahippocampal gyrus, substantia nigra, putamen, and thalamus (
50–
53). In addition, the kisspeptin system participates in several circuits within the limbic system that mediate anxiety, fear, other negative emotions, and olfaction (
52–
55).
The role of kisspeptin in fear has not been explored in many species. However, some studies of zebrafish have presented preliminary evidence supporting the notion that kisspeptin can decrease fear responses possibly via the serotonergic circuit (
56–
58). The serotonergic system has also been investigated in order to examine kisspeptin’s modulatory functions in emotion. In rodents, intraventricular administration of kisspeptin (dose dependent) overturned immobility, climbing, and swimming frequency during an experimental swimming test, confirming its potential neuromodulatory actions (
59).
Kisspeptin has also been implicated in anxiety and the reward pathways. Anxiety triggers stress hormones as regulated by the hypothalamic-pituitary-adrenal axis. However, this response is not affected by peripheral kisspeptin administration in animals or humans (
55,
60). Interestingly, kisspeptin signaling in the hypothalamus is reduced by stress-induced plasma corticosterone in rodents, indicating a possible connection between the endocrinological aspects of anxiety and stress responses (
53,
61). Additionally, kisspeptin appears to diminish the physiological effects of morphine, suggesting its possible involvement in the regulation of mesocorticolimbic dopaminergic activity (
62,
63). The centers for reward and addiction are functionally related to kisspeptin expression (
64). The dopaminergic projections connecting the ventral tegmental area and the nucleus accumbens have long been linked to the regulatory mechanisms of locomotion in rodents (
65,
66). Kisspeptin attenuates locomotion by possibly exerting a neuromodulatory role in the mesocorticolimbic dopaminergic pathways (
62).
The prefrontal cortex is an area known to express kisspeptin receptor mRNA in humans (
52). A recent study in which kisspeptin was administered peripherally to healthy male subjects revealed an increase in the activity of the prefrontal area (as measured by using functional MRI) in response to negative-evoked visual stimuli. Additionally, the investigators indicated a reduction in negative mood as measured with psychometric tests (
55). Taken altogether, these findings suggest that the kisspeptin circuitry and signaling responses may have a role in the modulation of mood, anxiety, and reward, by possibly influencing various structures of the limbic and paralimbic networks (
53).
Several limbic and paralimbic structures are linked to sexual processing mechanisms in the brain (
53). Human studies have indicated that the sexual processing centers in the cingulate and thalamus (cognitive), amygdala and insula (emotional), and putamen and precentral gyrus (motivational) are all involved in the mechanisms leading to the autonomic responses necessary for the induction of sexual behaviors. The kisspeptin neuropeptide and the KP neural circuitry appear to exert an intriguing neuromodulatory role in related neural circuits (
55,
67). The exact physiological mechanisms by which kisspeptin modulates sexual behaviors beyond GnRH inductions remain a matter of speculation. However, the kisspeptin signaling pathways, including potential interactions (i.e., anatomically, CN 0), are complex and warrant further study in relation to these behaviors (
53).