Treatment adherence is a significant problem for patients with bipolar disorder. Some studies suggest that as many as 60 percent of bipolar patients are at least partially nonadherent to medications.
There is evidence to suggest that long-acting injectable (LAI) antipsychotics may improve outcomes in these patients, but the only LAI medication approved to treat symptoms of bipolar I disorder is LAI risperidone. The results from a phase 3 trial published January 31 in the Journal of Clinical Psychiatry suggest that patients with bipolar I disorder may also benefit from a once-monthly LAI form of aripiprazole.
The study was funded by Otsuka Pharmaceutical Development and Commercialization and H. Lundbeck A/S, makers of AOM 400 (aripiprazole once-monthly 400 mg). The medication is indicated for schizophrenia only, but the companies are looking to expand the drug’s indication to include bipolar I disorder.
For the current study, Joseph Calabrese, M.D., director of the Bipolar Disorders Research Center at Case Western Reserve School of Medicine, and colleagues examined the effectiveness and safety of AOM 400 in patients with bipolar I disorder who were experiencing a manic episode.
The study was divided into multiple phases that required patients to first be stabilized on an average daily dose of 20.4 mg of oral aripiprazole before receiving an injection of AOM 400 every four weeks. A total of 266 patients who met stability criteria for eight weeks or more (Young Mania Rating Scale [YMRS] total score ≤ 12, Montgomery-Asberg Depression Rating Scale [MADRS] total score ≤ 12, and no active suicidality) were then randomized to receive injections of AOM 400 or placebo every four weeks for 52 weeks.
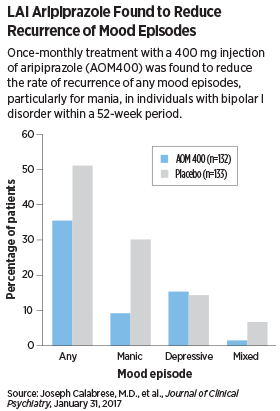
A total of 64 of 133 (48.1 percent) participants in the AOM 400 group and 38 of 133 (28.6 percent) participants in the placebo group completed the study. The primary measure outcome was the time from randomization to recurrence of any mood episode.
The authors found that significantly fewer patients in the AOM 400 group (26.5 percent) experienced any mood episode compared with the placebo group (51.1 percent), especially for mania. The LAI aripiprazole group was significantly more likely to report weight gain, akathisia, insomnia, and anxiety than the placebo group.
“These efficacy results are consistent with the known efficacy of oral aripiprazole monotherapy in the maintenance treatment of BP-I [bipolar I disorder],” wrote the study authors.
In addition, “tolerability was similar to that previously observed in patients with schizophrenia who are treated with the [LAI] formulation,” Calabrese told Psychiatric News. He noted that additional studies on the potential benefits of LAIs in bipolar I disorder are needed, including comparisons between oral formulations and commercially available LAIs.
“This was a well-designed … trial demonstrating superior efficacy of aripiprazole LAI for relapse prevention in patients with bipolar disorder,” Christoph Correll, M.D., a professor of psychiatry at Hofstra Northwell School of Medicine told Psychiatric News. “Having a second LAI approved for the maintenance treatment of bipolar disorder will be an important advancement and addition to our treatment armamentarium,” added Correll, who not was not involved with the study.
In November 2016, the Food and Drug Administration agreed to review a Supplemental New Drug Application submitted by Otsuka to expand indications of AOM 400 to include bipolar I disorder. The agency has set of target date of July 28 to complete the review, according to an Otsuka press statement. ■
An abstract of “Efficacy and Safety of Aripiprazole Once-Monthly in the Maintenance Treatment of Bipolar I Disorder” can be accessed
here.