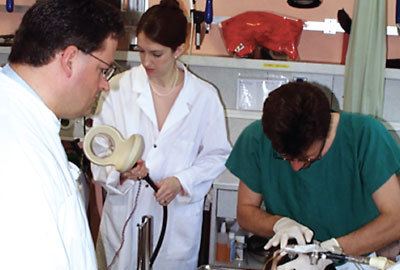
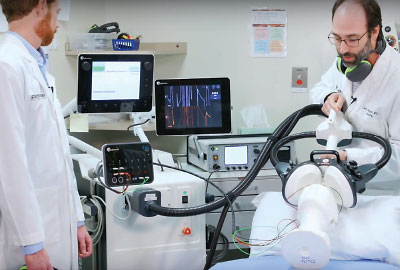
In May of 2000, Sarah Lisanby, M.D., then an assistant professor of psychiatry at Columbia University, was flying over to Bern, Switzerland, to test out a new tool in the fight against depression. She had spent the previous five years building and testing a device that used strong magnetic waves to stimulate controlled seizures in the brain. The hope was that this new approach to neuromodulation might provide severely depressed patients welcome relief from their symptoms without some of the risks associated with electroconvulsive therapy (ECT), which uses electricity to stimulate controlled seizures.
Lisanby’s colleague Thomas Schlaepfer, M.D., had identified a young woman with treatment-resistant depression who lived in Switzerland and was willing to undergo the experimental procedure. So, with a suitcase full of spare machine parts, Lisanby set out to conduct the first in-human trial of magnetic seizure therapy (MST).
The results of that trial, which were published 20 years ago this spring, were promising, setting magnetic seizure therapy on a long clinical journey that may soon reach fruition.
Magnetic Pulses Have Advantages
The research by Lisanby, now the director of the Noninvasive Neuromodulation Unit at the NIH Clinical Center, followed decades of work by others to increase options for patients with treatment-resistant depression. Lisanby is also the director of the Division of Translational Research at the National Institute of Mental Health (NIMH).
“Attempts to modify how ECT is given are nearly as old as ECT itself,” she explained. By adjusting the amount of current delivered and changing where the electrodes are placed on the scalp, researchers looked for ways to increase the efficacy and reduce the side effects of ECT, including short-term memory loss. One of the most significant safety advances in ECT practice was shifting the placement of electrodes from bilateral (one on each side of the scalp) to unilateral (one on the right side and one on the center top of the head), she noted.
“But physics limits how far we can advance with electric stimulation,” Lisanby said. Electrical currents meet heavy resistance and diffuse as they pass through skin and skull, thus making it difficult to target a specific region of the brain. Additionally, excess electrical energy spills out to surrounding brain regions, which leads to side effects.
In contrast, magnetic waves can pass through biological tissue unimpeded.
The idea of triggering brain seizures via magnetism was conceptualized in the early 1990s by Columbia’s Harold Sackeim, Ph.D., one of Lisanby’s mentors and a leading authority on ECT. At that time, a novel approach known as transcranial magnetic stimulation (TMS) was making headlines as a potential depression treatment. Though TMS relied on low-level magnetic energy to “gently” modulate brain activity, these pulses were found to induce seizures in rare instances. Sackeim reasoned that stronger magnetic pulses could induce more focalized seizures and mimic ECT’s antidepressant effects with fewer unwanted side effects.
MST is not without risks, as patients who receive the procedure report transient scalp pain and headaches, said Shawn McClintock, Ph.D., an associate professor of psychiatry at the University of Texas Southwestern Medical Center (UTSW) who studies neurostimulation therapies. “But the risk of short-term amnesia and other cognitive problems that have contributed to ECT’s stigma is far less.”
MST Hurdles Left to Clear
In the 21 years since Lisanby successfully treated her first patient with MST, the technology has undergone a steady if plodding progression toward clinical use. Numerous small studies, including a couple of head-to-head comparisons, have shown MST is about as effective as ECT at reducing depressive symptoms with fewer cognitive side effects. Some research centers, notably the Temerty Centre for Therapeutic Brain Intervention in Toronto, have also begun testing whether MST might be effective in patients with treatment-resistant bipolar depression or schizophrenia.
Despite more than 20 years of positive data and the approval of TMS for treatment-resistant depression in 2008, experts say it will likely be years before MST machines appear in clinics.
“MST requires a different device and uses different frequencies than TMS, so it is essentially a brand-new therapy and not a modified form of TMS,” said McLintock. “Seizure therapy is also an intensive treatment, so you want to proceed with the highest rigor, especially when it comes to safety, even if it takes more time.”
Another factor contributing to delays in moving MST to the clinic is that the medical device industry has not invested in this new technology as they did with TMS, Lisanby acknowledged. Currently, only MagVenture (based in Denmark) builds MST devices, and the company does not have the capital to sponsor large, multicenter studies (though they have donated machines for clinical research).
“NIMH recently stepped up to the plate and agreed to support a phase 3 trial” evaluating MST for treatment-resistant depression—a pivotal step for clinical approval, Lisanby explained. The trial is known as CREST-MST (for Confirmatory Efficacy and Safety Trial of Magnetic Seizure Therapy for Depression). The multicenter trial will be led by Daniel Blumberger, M.D., the director of Toronto’s Temerty Centre, and Carol Tamminga, M.D., the Lou and Ellen McGinley Distinguished Chair and the McKenzie Chair in Psychiatry at UTSW.
Comparison Trial Underway
As the director of the largest ECT service in Canada, Blumberger knows firsthand that ECT is the most effective treatment for severe treatment-resistant depression. He also knows it is the treatment option most refused when suggested to patients.
“If we could offer patients an alternative, we could help so many more,” he said. The same belief was held by the Centre’s prior director, Jeff Daskalakis, M.D., Ph.D., who in 2011 led the effort to obtain an MST device and funding to start clinical trials in Canada.
A decade later, the Temerty Centre has become a leader in clinical neuromodulation research and is a fitting choice to lead CREST-MST. Blumberger recently oversaw a head-to-head study that ultimately led to the FDA clearance of a new, rapid form of TMS known as theta-burst stimulation.
The CREST-MST trial is enrolling 260 adults with treatment-resistant depression at both the Temerty Centre and UTSW and providing them with either ECT or MST in a random and blinded fashion. (Both procedures are done under anesthesia so patients are not aware of their treatment; the clinicians assessing patient recovery will also be unaware of the treatment patients are receiving.) Blumberger told Psychiatric News that the trial has two primary goals. “The intent is to see whether MST is as clinically effective as ECT in terms of depression improvement and whether it has a better cognitive side-effect profile,” he said.
The trial had enrolled about 25% of participants when the COVID-19 pandemic hit last spring. Enrollment had to be put on hold as research activities and patient volumes were limited at both participating centers. Both sites have since reopened, and Blumberger is hopeful that the study can get back to full speed. He is also looking to re-launch another trial with his colleagues in Vancouver and London, Ontario, which is comparing MST and ECT for bipolar depression.
“When all the data are in, I am hopeful MST will meet the bar for regulatory approval and within a few years become a new option for treatment-resistant depression and potentially other refractory psychiatric disorders,” he said.
‘One Size Does Not Fit All’
If MST does emerge as a safer alternative to ECT, will that spell the end for the original neuromodulation therapy?
Lisanby won’t rule anything out but thinks it’s most likely that MST will become another option for patients with treatment-resistant depression rather than a replacement for ECT. She noted that despite the consensus that unilateral ECT was a tremendous advance over bilateral ECT, some patients still receive the latter procedure. “Patients who qualify for ECT have severe and treatment-resistant depression, so we want as broad a range of options as possible.”
“One size does not fit all,” agreed McClintock, who will also be working on CREST-MST. “As the field advances, we may find that ECT might be better in certain cases, such as patients with psychotic symptoms or suicidal ideation. That’s why we need to find biomarkers that may suggest which treatment would work best for a given patient.”
A key element of personalizing these brain stimulation procedures is to understand how they exert their effects—the mechanisms of ECT and MST are still somewhat of a black box. One of the focuses of McClintock’s research is using neurocognitive and neuroimaging tools to measure the changes in the brain after therapeutic seizures are induced. “We need to know why these treatments are doing what they are doing,” he said. ■
The details of the first MST trial, “Magnetic Seizure Therapy of Major Depression,” is posted
here.
More information on CREST-MST is posted
here.