Unlike many other chronic diseases, mental disorders often have an early onset affecting children and youth. As a result, if left untreated or insufficiently treated, they can rob individuals of the opportunity to live productive and satisfying lives. In the April 2015 issue of the journal Psychological Medicine, Holly Erskin, Ph.D., and colleagues (1) reported that mental disorders are the leading cause of disability in young people worldwide. By 2030 the associated annual global costs of mental disorders are estimated to be $6 trillion, according to Vikram Patel, Ph.D., and colleagues in the October 2018 issue of The Lancet (2). Mental disorders also magnify the risk of multiple other chronic conditions including heart disease, as reported by Ashton Teng, M.S., and colleagues in the June 2020 issue of the Journal of Psychiatric Research (3). Just as ominous is the possibility that exposure to the “wrong” treatment over time may itself change the brain, making episodes worse and more frequent. Beyond the personal challenges, these disorders significantly impact loved ones, caregivers, and society in general.
Current treatment modalities can be remarkably effective in mitigating the burden of symptoms of many mental disorders, yet finding the right treatment for an individual can be a long, fraught process during which symptoms can increase the risks associated with other health conditions. New and emerging treatments are available to those for whom multiple trials of conventional therapies have failed, but we currently lack strategies for selecting effective treatments for each individual in a timelier manner that limits the need to wait for multiple tries and failures of ineffective treatments.
Precision psychiatry presents a new path forward for connecting each patient with the exact treatments he or she needs, sooner, by using advances in science and technology. The emerging field of precision psychiatry seeks to develop therapeutic approaches tailored for specific individuals with a specific constellation of health issues, characteristics, strengths, and symptoms. Through these approaches, we hope to reduce the burden of disorder experienced by patients, families, and wider communities. We have a rich system of symptom classifications as defined by the
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition-Text Revision (DSM-5-TR) and the “
International Classification of Diseases and Related Health Problems, 10th revision, Clinical Modification (ICD-10-CM)”. These systems provide standardized nomenclature. The rapid accumulation of neuroscience insights has given us the opportunity to complement these systems with neurobiological information and psychosocial factors that interact with neurobiology and symptom expression. Neuroscience insights offer new ways to account for the heterogeneity within and across mental disorders and to consider treatment selections accordingly. These insights link clinical phenotypes to an individual’s biosignature (or biotypes), opening the possibility that treatments can be adapted and selected to target specific clinical features and biotypes. In some cases, underlying neurobiology may align with
DSM- or ICD-based classifications. In other cases, it may cut across diagnostic classifications or identify unforeseen, novel biotypes.
For precision psychiatry to make a difference in patients’ lives and be useful to clinicians, it must be driven by clinical translational goals. We can envision a future in which clinical decision-making is complemented by tools and measures that help to diagnose individual clinical biotype profiles and tailor treatments to these profiles. Realizing this future will involve an ongoing integration of findings from basic and clinical neuroscience, as well as population-level data from clinical practice and new forms of clinician-researcher partnership. No single subdiscipline will have all the required domain knowledge.
In our book,
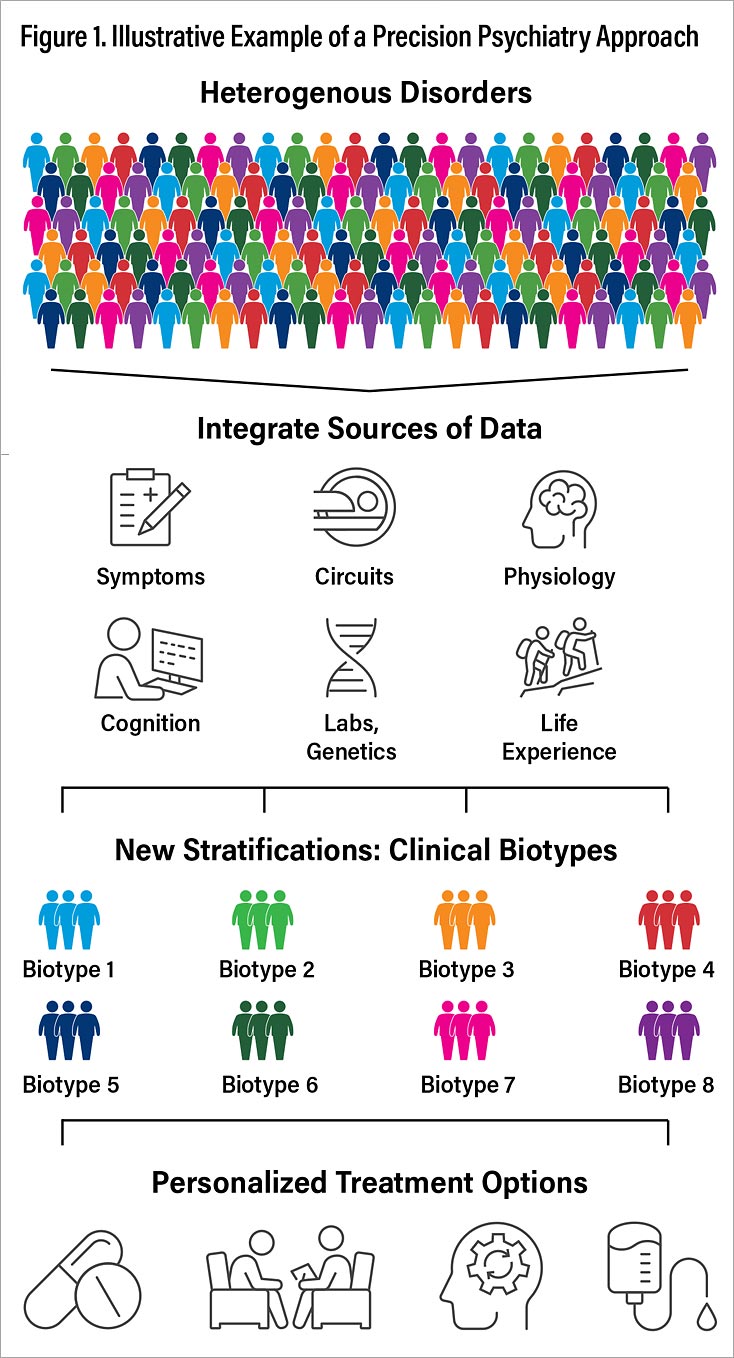
Precision Psychiatry, Laura Hack, M.D., Ph.D., and I harnessed the expertise of more than three dozen contributors in diverse areas of interest—including neuroimaging, electrophysiology, neurocognition, inflammatory markers, pharmacogenetics, behavioral science, machine learning, pharmacotherapy, and neuromodulation and neuroscience in psychiatry education—to examine the current state of precision medicine in psychiatry and explore future areas of advancement (Figure 1). Case illustrations are included for each area of advancement. Our hope is that educators, trainees, and clinicians will find the latest research in precise classification, treatment planning, and early identification across a spectrum of psychiatric disorders. We also aimed to provide a foundation for a future in which one-size-fits-all treatments are replaced by modalities that are optimized for individual patients across all stages of a disorder.
It’s Psychiatry’s Turn
The national focus on precision medicine has been driven by the urgent need to revolutionize how we improve health and treat disease in general. The emerging emphasis on precision psychiatry in particular is driven by the increasing urgency to address the enormous social burden of psychiatric disorders and close the translational gap between research and practice.
U.S. federal initiatives have been launched to make progress in these areas, including the National Institute of Mental Health Research Domain Criteria initiative, outlined by then National Institute of Mental Health Director Thomas Insel, M.D., in the
American Journal of Psychiatry in April 2014
(4); the “
BRAIN Initiative”; and the “
Human Connectome Project”. More recently, Congress passed the “
Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019”, which instructs the Veterans Administration to implement the Precision Medicine for Veterans Initiative to identify and validate brain and mental health biomarkers among veterans, with specific consideration for depression, anxiety, PTSD, bipolar disorder, and traumatic brain injury. Under this initiative, the methods should include brain structure and function measurements, such as functional magnetic resonance imaging (fMRI) and electroencephalography, and coordinate with additional genetic and biomarker methods of analysis utilized in the Million Veterans Program of the Department of Veterans Affairs.
“Precision” treatment is not an entirely novel approach in health care. An analogy can be made between new advances in precision psychiatry and those in other areas of medicine that are further along in closing the gap between scientific and technological advances and the clinical applications of those advances. Just 74 years ago, cardiovascular medicine could not measure the unobservable aspects of heart structure and function due to the lack of tools such as fluoroscopy, multidetector computed tomography, ultrasound, magnetic resonance imaging, cardiac perfusion scintigraphy, echocardiography, and positron emission tomography. Physicians would have attempted to personalize their assessments to each patient but they were not able to observe the heart’s behavior or how it was related to observable symptoms. Today, this ability is taken for granted.
The shift began in 1948 with the Framingham Heart Study, which was inspired by Franklin Delano Roosevelt’s death from cardiovascular disease and the use of multiple “wrong” treatments. The Framingham study spawned the assessment of standard vital signs as well as a subsequent range of imaging techniques capable of linking precise insights about the organ of interest (the heart) to treatment indications and even prevention. It is now routine to take serial images to provide a baseline from which to assess each individual’s recovery and his or her subsequent risk for relapse. These measurements also inform the development of a personalized plan that considers the whole individual, including lifestyle and diet, alongside medical interventions. For example, echocardiography can be used to identify heart muscle and valve function and to indicate specific treatments (for example, heart valve surgery) and types of arrhythmias (for example, too fast, too slow, irregular); the angiogram can confirm the presence of blockage of blood flow (for example, thrombus causing stroke and myocardial infarction) and indicate other treatments such as clot removal, medications, and lifestyle modification.
Precision oncology is now an established form of cancer treatment, in which the unique biology of a patient’s disease is assessed and used to make tailored and targeted treatment selections. Scientific and technical advances in personalized genomics have revolutionized care by enabling physicians to test each patient’s cancer to find out if a certain type of treatment will be effective, to learn from the patient’s genetic code whether he or she will be able to cope with the treatment, and to undertake genetic testing to identify future risks for cancer. Because the testing of the genetic makeup of tumors has become a relatively quick and low-cost procedure, it has rapidly become one of the catalysts for progressing personalized cancer care. To enhance precision, genetic information is increasingly being integrated with the imaging of tumors to further characterize each patient and tailor treatment needs accordingly.
The Meaning of ‘Precision’ in the Context of Psychiatry
What if the principles of precision medicine already at work in fields such as cardiology, oncology, and infectious disease—which use biomarkers combined with clinical features to select treatments for specific patients—were applied to psychiatry?
At its core, precision in the context of psychiatry is an approach that focuses on understanding the underlying neurobiological mechanisms that cause the symptoms of psychiatric disorders. Using this understanding, we have the foundations for developing treatments that can target these mechanisms, which in turn will address previously untreatable aspects of psychiatric disorders by providing more specific symptom control and possibly altering the trajectory of the disease process. Rather than focusing on diagnostic categories (that is, a syndrome-based treatment approach), the emphasis is on solutions that span pharmacological, behavioral, neuromodulation, and novel therapeutics and can alter the specific biological processes that lead to the manifestation of symptoms and phenotypes. This is critical because mental disorders are collections of many different processes that interact across time to develop the unique pattern of symptoms that are experienced by each individual.
In Precision Psychiatry, we considered at least three components of precision psychiatry that can be described by at least three interchangeably used terms: stratified medicine, personalized medicine, and precision mental health. “Stratified medicine” focuses on identifying subgroups of patients who will benefit from treatments as a step toward a fully personalized approach that tailors treatments to individual people. “Personalized medicine” focuses on harnessing new advances in genomics to select treatment options with the greatest likelihood of success, but now it is being expanded to include circuit- and other neuroscience-based assessments. “Precision mental health” can be thought of as a new frontier, expanding on precision medicine to a wider concept of health and prevention that goes beyond a focus on disease.
An assumption inherent in this perspective is that precision will rely on measurement. The adjective “precision” in the term precision psychiatry conveys this foundation in measurement. It emphasizes the goal of exactness in measurement that, eventually, may allow us to personalize our approaches.
A related assumption is that, in our field of psychiatry, the foundation must necessarily involve the organ of interest: the brain. Of course, we must always remember that brains develop and function in the context of predisposing factors and a dynamic interplay with genetic dispositions and ongoing life experience.
From this view, there are three main goals for developing a precision approach to psychiatry:
•
Precise classification (that is, a specific understanding of the pathophysiology of each individual patient—what has gone wrong?). Precise classification hinges on the identification of subtype profiles that map neurobiology and symptoms, taking into account life experience, and are relevant to guiding treatment choices. As noted earlier, these profiles and biotypes may in some instances align with our current diagnostic categories, but they may also cut across them or represent new subgroupings.
•
Precise treatment planning (that is, tailoring treatment plans in a personalized manner—how can we fix what has gone wrong?). Although many effective treatments are available, finding the right treatment for the individual patient is largely a matter of trial and error, and treatment selection is not linked to a precise understanding of an individual patient’s neurobiology.
•
Precise prevention (that is, targeted and tailored prevention strategies—how can we keep things from going wrong?).
A major challenge for precision medicine in psychiatry is that psychiatry does not yet use measurements in routine clinical practice. This is the case for measurement of diagnostic symptoms and for tracking the equivalent of vital signs, imaging the organ of interest (the brain), or lab-based genetic testing. It is not surprising that psychiatry is new to the game regarding precision medicine given the complexity of the brain and the behaviors of interest. The following sections of this article outline two approaches for closing the clinical translational gap, as well as case illustrations.
Toward a Clinically Applicable Circuit Approach to Precision Psychiatry
One approach to closing the translational gap focuses on promising research showing that neuroimaging may be useful for identifying specific brain circuits informative of antidepressant response.
This approach conceptualizes mental illnesses as disorders of brain circuit function. Treatment strategies could target an individual’s circuit signature (or biotype) in a more granular fashion and allow for higher adaptability to symptom heterogeneity.
Beginning in the 1990s, advances in imaging modalities such as fMRI have enabled the quantification of the human brain with sufficient spatial and temporal resolution to identify relationships between psychiatric illness and dysfunction in distributed brain circuits. Circuits—or brain networks—reflect the functional neural organization of the brain. Circuits integrate across different levels and measures of brain function, but still reflect its complexity. Researchers have identified a universal neural circuit architecture that encompasses intrinsic circuits that are not specific to tasks and partially distinct circuits that are evoked by reward-related, affective, and cognitive processes.
Clearly, there is extraordinary detail in such circuit organization. Nonetheless, substantial progress has been made toward characterizing biotypes for depression, anxiety, schizophrenia, and attention-deficit/hyperactivity disorder.
To make headway toward a clinically applicable taxonomy, I have focused on six circuits that show dysfunction in depressive and anxiety disorders in meta-analyses and/or at least two well-powered studies, as outlined in my article “
Precision Psychiatry: A Neural Circuit Taxonomy for Depression and Anxiety”
(5) in the April 2016 issue of
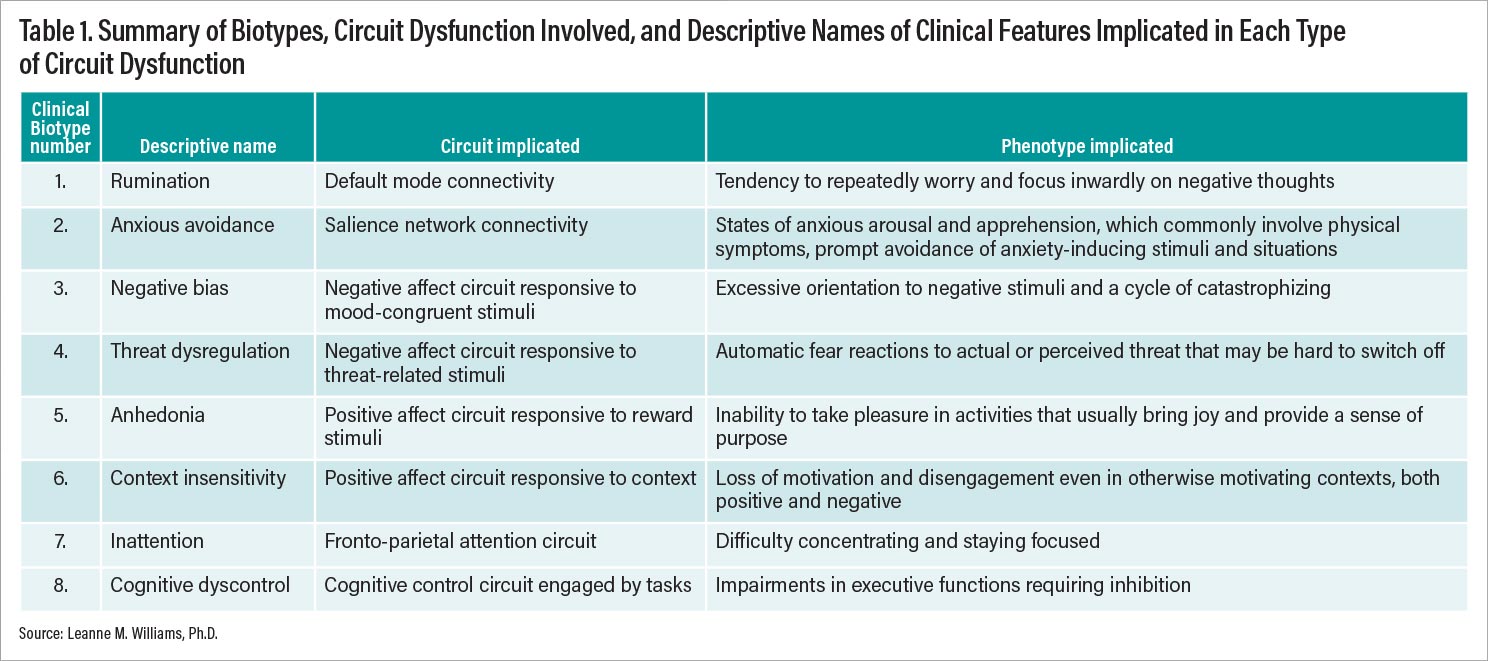
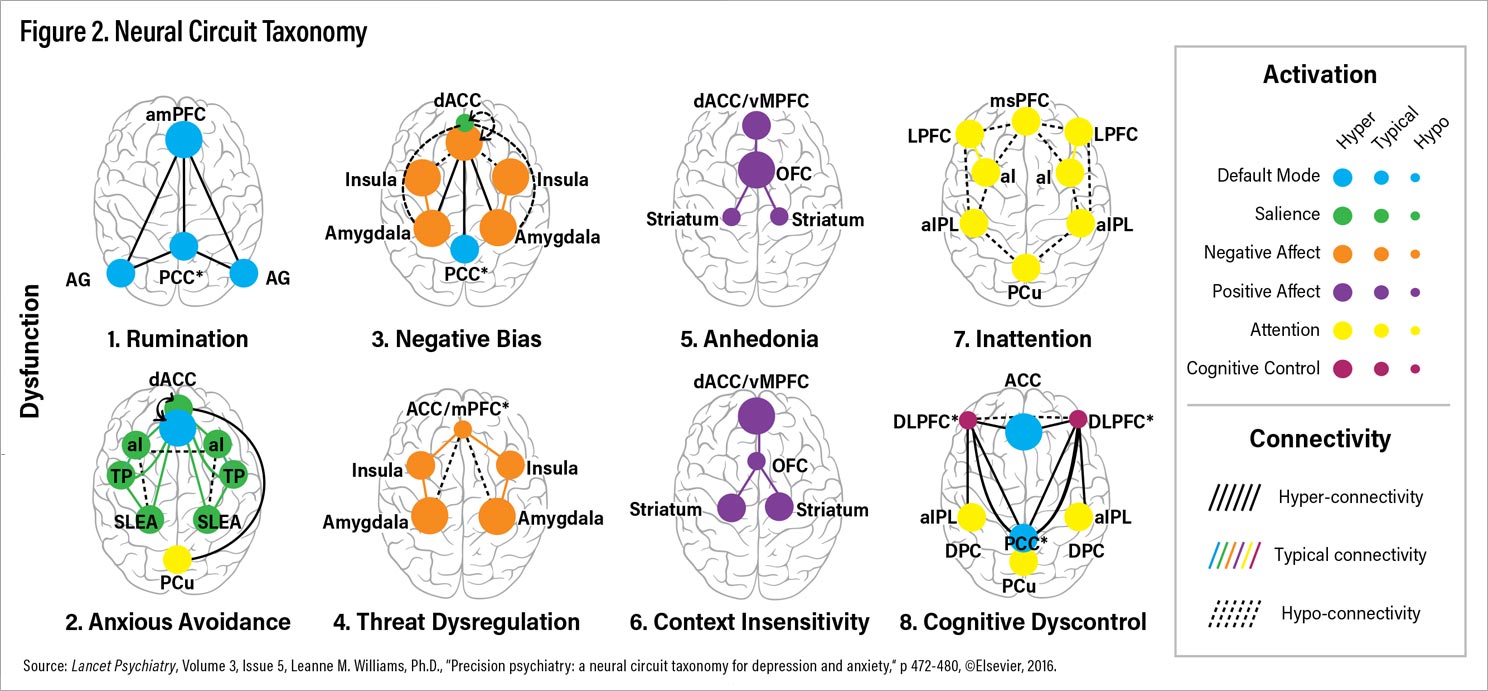
Lancet Psychiatry. Based on current knowledge, I have proposed at least eight distinct ways that brain circuits could get disrupted or stuck and that are putative biotypes for depression and anxiety (Figure 2). These biotypes are referred to by both number and descriptive names that reflect the clinical features implicated in each type of circuit dysfunction (Figure 2; Table 1).
To quantify the circuit functions that underlie these putative biotypes, steps have been made toward developing and testing a reproducible system that could be applied to fMRI data from individual patients. This system and the resulting circuit clinical scores are reported by Goldstein-Piekarski et al. in the July 2021 issue of Biological Psychiatry (6). The dysfunctions measured by these circuit scores show promise for helping select among currently approved treatments. As examples of this promise, we can consider convergent findings across large-scale precision medicine treatment trials and biotype initiatives, including PReDicT (Predicting Response to Depression Treatment), EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care), and iSPOT-D (international Study to Predict Optimized Treatment in Depression).
For example, findings derived from task-free fMRI show that hypoconnectivity within the default mode network, particularly with the rostral anterior cingulate (rACC) region, identifies patients who are unlikely to respond to typical first- and second-line antidepressants. Emerging evidence suggests that transcranial magnetic stimulation may be one new treatment that regularizes default mode connectivity (see Case Example #1). Relatively greater connectivity of the rACC within the default mode networks and its connections with other networks, including the anterior insula-defined salience network, predicts better recovery on antidepressants. Connectivity between these regions also differentially predicts response to antidepressants versus cognitive-behavioral therapy.
In response to emotion tasks, hyporeactivity of the amygdala within the negative affect circuit has been found to differentially predict response to selective serotonin reuptake inhibitors (SSRIs) and a selective serotonin-norepinephrine reuptake inhibitor. Correspondingly, activation of reward circuit regions have been found to differentially predict response to an SSRI versus a norepinephrine-dopamine reuptake inhibitor.
In patients who have anhedonia and do not benefit from monoaminergic reuptake inhibitors, novel treatments that target regions of the reward circuit and specific dopamine or kappa opioid receptors have been found to alleviate both anhedonia and blunted reward circuit activation (see Case Example #2). Because the circuits implicated in these findings are relevant to many other mental disorders beyond depression, they may have wider applicability as targets for treatments in other areas.
Toward Clinical Implementation of Pharmacogenetic Decision Support Tools
A second key area of progress in precision psychiatry is the development of pharmacogenetic decision support tools. These tools are more advanced as commercially available tools focused on antidepressant drug prescribing.
The APA Task Force for Novel Biomarkers and Treatments, led by Charles Nemeroff, M.D., Ph.D., summarized gene-drug evidence for commercially available pharmacogenetic tools for antidepressant prescribing available at the time of its 2018 report (“
Task Force on Gene Testing for Antidepressant Efficacy Concludes Tests Not Yet Ready for Widespread Use”). Due to limitations of study designs and available data, the task force concluded that there is “insufficient evidence to support pharmacogenetic testing in practice.” It was noted that tests involving CYP2D6 might be worthwhile in helping to guide dosing strategies to reduce side effects.
In Precision Psychiatry, contributing experts provide an outline of the current gene-drug knowledge underpinning the provision of pharmacogenetic testing in psychiatry, with clinical cases to illustrate potential utility in practice (see Case Example #3).
A report from the Precision Medicine in Mental Health Care Trial (PRIME Care) published July 12 in JAMA (7) indicates that provision of pharmacogenomic testing for drug-gene interactions, using the commercial test PGx, reduces prescription of medications with predicted drug-gene interactions compared with usual care. PRIME Care findings are from 1,944 veteran patients at 22 sites across the United States. This report also estimates that 20% of patients in usual care may have problematic gene-drug interactions that could be picked up with testing.
Bridging the Clinical Translational Approach
Bridging the gap between discovery and the clinical translation of precision requires intentional integration across clinical, research, education, and policy endeavors. Although this is a big goal, we could learn from other areas of medicine and start with areas in which the “cost/benefit” ratio may be high, such as with patients who are nonresponsive to available treatments and/or experience adverse events.
In Precision Psychiatry, we identified several areas in which advances are needed, though our list was by no means exhaustive. First, we need clinically applicable quantitative metrics and norms for the measures studied in precision psychiatry research. Our circuit metrics are one of many possible examples. Second, we need multisite, longitudinal cohort studies that deploy standardized protocols in clinical practice settings, with multiple measurement modalities, and the opportunity to evaluate prospective assignment to treatments based on emerging precision psychiatry tools. Third, it is essential that we develop the infrastructure to enable the investigation of a wider array of interventions in parallel using the same measures and precision psychiatry tools. Typical research designs are funded to focus on one treatment at a time, in serial. We lack data to directly compare across treatments to determine which measures and tools are specific to which treatment, indication, and biotype.
A common question that arises when discussing precision psychiatry approaches is “What is the cost?” Comprehensive cost-effectiveness and cost savings analyses are essential. As summarized in the report of the APA Task Force for Novel Biomarkers and Treatments, the cost-effectiveness and savings evaluated over one year ranges from annual averages of $1,300 to $5,174, and the estimated increase in quality-adjusted life-years per patient is 0.3. A further context for cost evaluations might be the balance between upfront testing costs relative to longer-term costs of chronic disability and health care utilization associated with untreated or insufficiently treated mental disorder beyond a one-year time frame. Of course, these ideas presuppose the availability of tests that get more people effectively treated sooner than is the case now.
Other clinical factors to consider are new opportunities for patient engagement, shared decision-making, and education. As outlined in Chapter 1 of our book, several of these opportunities were observed through a discovery clinic for neuroscience-informed precision psychiatry launched at Stanford in 2013. Spontaneous patient feedback highlighted potential benefits for patient engagement. Patients reported that “seeing their own brain” and profile of measurements helped them to resolve some of the stigma around their experience of having a mental disorder and gave them a renewed motivation to engage in treatment planning. Patients referred to the value of tangible measures to help them participate in treatment planning and to understand the reasons for choices. In this discovery clinic example, test data provided sources of information to complement clinical judgment and clinical decisions.
The promise of precision psychiatry to provide additional tools in the clinical toolkit aimed at complementing clinical judgment also relies on training and education that meets the needs of busy clinicians. There is a need to equip practitioners with terminology and training in neuroscience-based tools suited to integration with current decision-making practices. It is not viable for clinical practitioners to become domain experts, just as it is not viable for clinical neuroscientists to be experts in all content areas. Much of our evidence is derived from trials that are not integrated with clinical settings and workflows. Intentional collaborative approaches and consortia that rely on mutual clinical-research feedback are key to closing the gap between discovery and delivery into practice. ■
Author Disclosure Statement
The author receives royalties from APA Publishing and annual advisory board fees from One Mind Psyberguide and Laureate Institute for Brain Research and declares US Patents. App. 10/034,645 and 15/820,338: Systems and methods for detecting complex networks in MRI image data.