Researchers at Rockefeller University in New York believe they have found evidence that lecanemab may work by disrupting amyloid-beta’s ability to activate a part of the body’s blood- clotting network known as the plasma contact system. They described these findings in a brief report in PNAS.
How Plasma Contact System Works
The plasma contact system involves a series of biochemical reactions that are triggered in response to nearby tissue damage. While the plasma contact system can induce some clotting, it mainly facilitates the clotting process by dilating blood vessels and activating the immune system, explained lead study author Erin Norris, Ph.D., a research associate professor at Rockefeller.
Norris and study co-author Sidney Strickland, Ph.D., the Zachary and Elizabeth M. Fisher Professor in Alzheimer’s and Neurodegenerative Disease at Rockefeller, have been researching the contact system for years. Previous research by the pair has suggested a relationship between the activation of the contact system and memory impairment in people with Alzheimer’s disease and mild cognitive dementia. In addition, an
analysis of blood samples from Alzheimer’s patients demonstrated that amyloid-beta may be able to directly activate the contact system.
For this new study, Strickland, Norris, and colleagues set out to determine the specific type of amyloid-beta that triggers the contact system.
Many Faces of Amyloid-Beta
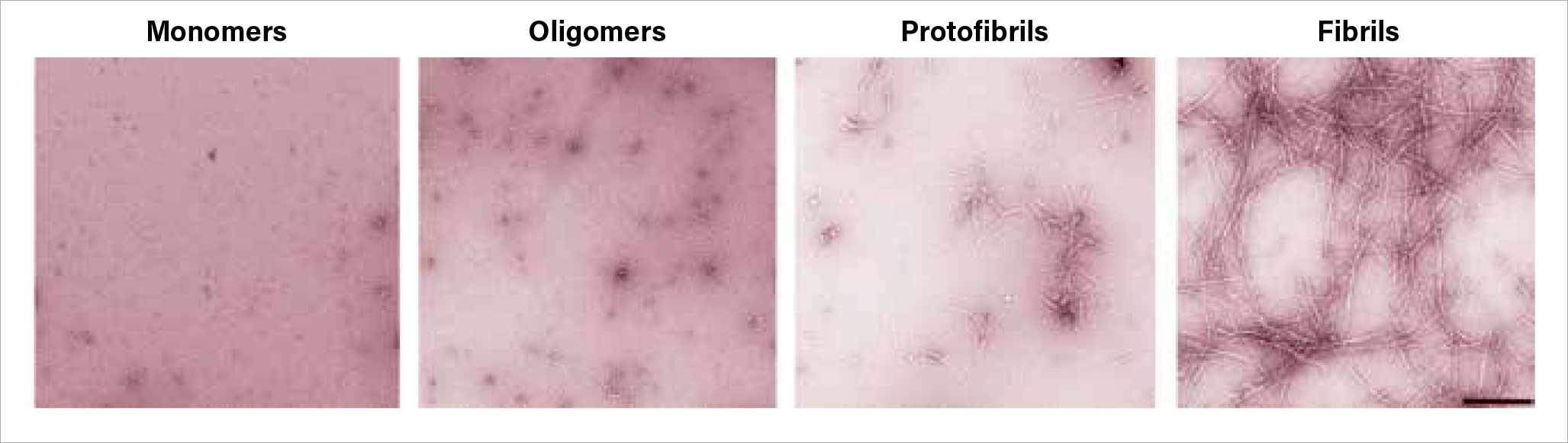
Amyloid-beta exists in four progressive configurations:
•
Monomers: free-floating protein fragments.
•
Oligomers: small clumps of monomers.
•
Protofibrils: rigid chains of oligomers.
•
Fibrils: insoluble nets of protofibrils.
While the fibril structures make up the plaques that are characteristic of Alzheimer’s, many researchers believe the protofibrils are the most toxic form of this molecule. For their analysis, the Rockefeller researchers created amyloid-beta structures of each type and added them to blood samples from healthy donors. They found that protofibrils were the only amyloid-beta form that activated the contact system.
It was known that the amyloid-beta antibody lecanemab preferentially attaches to amyloid protofibrils, rendering them inert until they are broken down by white blood cells. When the Rockefeller researchers added lecanemab to the blood samples along with the protofibrils, they found that the contact system was no longer activated.
George Perry, Ph.D., the Semmes Foundation Distinguished University Chair in Neurobiology at the University of Texas at San Antonio, said the findings advance what researchers understand of the biology of amyloid-beta, but cautioned against extrapolating the results too far.
“These experiments were done using assemblies of pure amyloid,” he told Psychiatric News. In the living brain, amyloid structures assemble around other proteins and molecules.
Additionally, Perry said that the demonstrated interaction may not be clinically relevant, as researchers continue to debate whether amyloid-beta protofibrils are inherently toxic in living brains. As he and others have noted, these antibodies can clear significant amounts of amyloid-beta fragments from the brain, yet patients do not get better.
Strickland and Norris acknowledged that the latest findings do not offer definitive proof that lecanemab’s clinical benefits are via blocking the contact system. However, the findings may explain why lecanemab has lower rates of side effects such as brain edemas than the related amyloid drug aducanumab, which was controversially approved in 2021.
As noted, the contact system promotes blood vessel dilation, which in turn can increase the risk of localized brain swelling and/or bleeding. By breaking down protofibrils, lecanemab can reduce contact system activity and risks of bleeding.
Aducanemab targets amyloid oligomers, which according to this study appear to have no direct impact on the contact system. A third amyloid therapy awaiting FDA approval, docanemab, directly targets the large, insoluble amyloid fibrils. This drug has also shown
higher rates of vascular side effects than lecanemab in clinical studies.
“There are so many things going wrong in Alzheimer’s disease, and contact system overactivation is just one small part,” said Norris, who has been working with Strickland on a diagnostic test to identify individuals with a dysregulated plasma contact system. “But if we can identify and address even one problem in a patient, that will help.”
This study was supported by grants from the National Institutes of Health along with the Robertson Therapeutic Development Fund, Samuel Newhouse Foundation, John A. Herrmann, and May and Samuel Rudin Family Foundation. ■