“The future is already here, it’s just not evenly distributed.” — William Gibson
Psychiatry is at a crossroads. On the one hand, it deals with what is arguably one of the most important subject matters there is: optimizing the functioning of the mind. This is increasingly important in our knowledge-based economy—and increasingly difficult, as people find their cognition, focus, and mindset taxed and compromised by 21st-century lifestyles.
On the other hand, psychiatry is still practiced in most places as 19th-century medicine, in a subjective, qualitative way, relying on what patients report and on the clinician’s impression and pattern-recognition skills, without the guidance of objective laboratory tests, electrophysiology, or imaging. What if cardiology was practiced that way today? There would be an outcry. Yet this is how we approach treating an organ that is arguably even more complex than the heart.
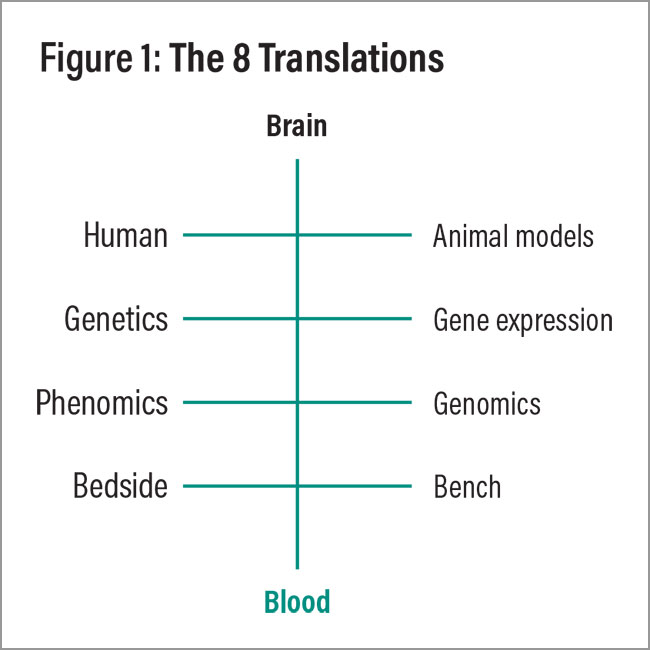
What can be done to improve things? The good news is that progress has been made in recent years to make psychiatry more like oncology (the field in which I did my graduate work) or cardiology (my favorite rotation in medical school)—including significant advances in genetics, biological markers, electrophysiology, imaging, and digital tools (Niculescu and Le-Niculescu, 2022). Translating them into routine clinical practice is an exciting opportunity that stretches ahead of us for the rest of this decade, bolstered by the exponential acceleration of AI (Figure 1). After more than 20 years in this field, I could not be more excited and energized.
Genetics in Psychiatry
Psychiatric genetics has been focused primarily on finding DNA mutations and variations that underlie, or at least are correlated with, disease. There are advantages and limitations to this approach.
The advantages include the fact that DNA is as easily accessible as having a drop of blood collected on a card from a newborn, or doing a mouth swab later. Also, DNA is (more or less) unchanged from birth—in fact from conception—so prenatal or early-childhood screening could be done before any disease manifests itself.
The limitations include that there is a big distance from DNA to phenotype (disease manifestation), because most mutations and variations have an extremely small effect. (There are some very rare mutations that do have a larger effect size in some families; these involve chunks of DNA that are multiplied or deleted, called copy number variants, or CNVs. Rare CNVs have been associated with increased risk of schizophrenia, autism spectrum disorder, and intellectual disability.) Plus, the variants identified to correlate with disease often have an unknown relationship to disease biology, and environmental effects are not factored in when looking at DNA.
As such, even stringing together a panel of mutations and variants in a so-called polygenic risk score (PRS) is not yet clinically informative or actionable. In psychiatric genetics, a PRS typically uses tens of thousands to millions of single nucleotide polymorphisms (SNPs), depending on the specific disorder, the scoring methodology, and the available genome-wide association studies (GWAS) data. Here is a general breakdown for key psychiatric disorders:
•
Schizophrenia: PRSs for schizophrenia often incorporate hundreds of thousands to millions of SNPs. The Psychiatric Genomics Consortium has identified more than 100 loci associated with schizophrenia, with broader scores using over a million SNPs for better predictive power.
•
Major depressive disorder (MDD): PRSs for MDD usually involve fewer SNPs than schizophrenia, given that MDD is more polygenic and the effect sizes of associated SNPs are generally smaller. Scores typically include hundreds of thousands of SNPs, especially with data from large consortia.
•
Bipolar disorder: For bipolar disorder, PRSs often include a high number of SNPs, with larger GWAS studies in recent years allowing for a PRS with hundreds of thousands of SNPs, although fewer than those used in schizophrenia scores.
•
Autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD): These conditions tend to have lower predictive power in PRS compared with schizophrenia or MDD due to smaller GWAS sample sizes and more complex genetic architectures, often incorporating fewer SNPs but still reaching into the tens of thousands.
•
Generalized anxiety disorder and other conditions: Anxiety-related PRSs are less well developed due to smaller GWAS, but they still typically use tens of thousands to hundreds of thousands of SNPs in recent studies.
The number of SNPs used in a PRS is generally selected to maximize predictive accuracy, balancing between including enough SNPs to capture polygenic effects and excluding SNPs with minimal effect sizes to avoid noise.
A weakness of such studies to date has been their emphasis on determining the statistical significance (p-value) of the SNP findings without trying to reproduce the findings in multiple independent cohorts to make sure they are real (Niculescu and Le-Niculescu, 2010). When that has been attempted, reproducibility has been very poor, likely due to overfitting of the initial results and the fact that there is a lot of variation between individuals at an SNP level (and much less so at the gene and biomarker levels, as we discuss below).
Various approaches to enrich the PRS in variants that have high functional/biological impact are underway, including by my group.
Biomarkers in Psychiatry
Biological markers (biomarkers) are molecules that reflect and track disease biology, clinical severity, and response to treatment (Niculescu and Le-Niculescu, 2022). They range from epigenetic markers (regions of DNA that are methylated or otherwise modified) to messenger RNA, or mRNA (coding for proteins, and reflecting gene expression), various other newly identified forms of regulatory RNAs, protein levels, protein modifications (such as phosphorylation and glycosylation), and metabolites (such as glucose, various lipids, some hormones, and neurotransmitters). They can be measured in saliva, blood, CSF, and even olfactory epithelium cells (Etyemez, et al., 2023).
The advantages of biomarkers are that they are tied more directly to biology, are closer to phenotype, have environmental effects factored in, and change in response to treatment. Most importantly, biomarkers have been much more reproducible to date than genetic GWAS findings, have a more pronounced effect, and are thus clinically actionable (Niculescu and Le-Niculescu, 2022) and already starting to be used in clinical practice. The limitation is that biomarker alterations may be detectable once the disease manifests itself, not prior to that in early childhood or prenatally, as DNA allows. Thus, they are less amenable to early screening and prevention.
mRNA biomarkers represent a sweet spot between DNA and phenotype, with genes and environment effects reflected. The mRNA of all the genes (the whole transcriptome) can be assessed in one fell swoop—in the past with microarrays (Niculescu, et al., 2017), now with modern sequencing technology (Bhagar, et al., 2024). Unlike with cancer, where you can biopsy a tumor, in psychiatry, you cannot readily biopsy the target organ (the brain) in live individuals. Because of this, my group has sought peripheral markers that are robust but also easy to use. We and others have focused on blood, and have been successful in identifying reproducible and predictive blood biomarkers for suicidality risk (Niculescu, et al., 2017; Bhagar, et al., 2024; Le-Niculescu, et al., 2013; Levey, et al., 2016; Niculescu, et al., 2015; Mamdani and Sequeira, 2023), stress (Le-Niculescu, et al., 2019; Dean, et al., 2020; Daskalakis, et al., 2024), anxiety (Roseberry, et al., 2023), mood (Le-Niculescu, et al., 2009; Le-Niculescu, et al., 2021), psychosis (Kurian, et al., 2009; Niculescu, et al., 2000; Hill, et al., 2024), memory disorders (Niculescu, et al., 2019), pain (Niculescu, et al., 2019), and even longevity (Rangaraju, et al., 2016).
In order to do that, my group took a careful, four-step approach: discovery, prioritization, validation, and testing. The signal is there, but it is like finding a needle in a haystack. The fact that it exists in the first place is due to the close brain-immune and brain-periphery bidirectional interactions, as well as to the fact that environment and medications affect gene expression not only in the brain but also in periphery.
Beyond RNA, at a protein level, two types of biomarkers traditionally have been studied:
•
Inflammatory markers: Inflammatory cytokines like interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-alpha (TNF-α) are elevated in some individuals with depression, schizophrenia, and bipolar disorder, suggesting immune system dysregulation.
•
Neurotrophic factors: Brain-derived neurotrophic factor (BDNF) levels are often reduced in patients with depression and other mood disorders, particularly during active depressive episodes.
A third type will be to develop tests based on the proteins that are encoded by the well-validated mRNA biomarkers described above. Such tests could lead to point of care and even at-home testing, as is done for COVID and pregnancy, among other things.
At a metabolite level, hormonal biomarkers traditionally have been studied. For example, cortisol, a stress hormone, is often dysregulated in individuals with depression, posttraumatic stress disorder, and other stress-related disorders, with altered cortisol levels linked to hypothalamic-pituitary-adrenal axis dysfunction.
In the future, metabolites of neurotransmitters (such as serotonin) based on biological pathways highlighted by the mRNA biomarkers mentioned may become validated and useful in clinical practice. I anticipate that metabolites will be the most powerful biomarkers, more so than mRNA and even proteins. As an analogy, in diabetes, the most powerful biomarker is glucose level, and for trait there is a protein, hemoglobin A1C. No mRNA biomarkers or PRSs are needed.
One area where biomarkers will have a great impact besides diagnostics and routine clinical practice is the development of psychiatric drugs. Relying on DSM classifications and legacy questionnaires has led to a relative lack of success in the development and successful approval of new neuropsychiatric drugs. Clinical trials combined with biomarkers would lead to more targeted drug development, with better outcomes and even the possibility of companion diagnostics, like with cancer. Most psychiatric disorders may end up stratified by molecular subtypes, again like in oncology.
An immediate impact of biomarkers is in the area of objective and quantitative assessment/diagnostics. That is the first step. Another area of interest is matching people to the right treatments—step two. Deciding what dose to use is step three, and that has been informed for a while by available cytochromeP450 pharmacogenetic DNA tests.
Electrophysiology in Psychiatry
Electrophysiology in psychiatry cuts both ways. There is its diagnostic use, with methodologies such as electroencephalogram (EEG) “biomarkers” that are starting to be used in clinical trials (Wu, et al., 2020), if not in clinical practice. The jury is still out. The distance between underlying biology and EEG signal may be a problem, although there are examples of successful uses in other fields, such as cardiology with its EKG.
Electrophysiology also has interventional uses, which range from the historical tradition of electroconvulsive therapy (ECT) to the more recent, targeted, and benign transcranial magnetic stimulation (TMS).
Neuralink has developed a futuristic two-way brain-computer interface (BCI), used so far for neurological disorders, but with tremendous potential for psychiatry. The pioneering work of neurologist Helen S. Mayberg, M.D., and colleagues with electrode stimulations in discrete brain areas for treatment-refractory depression has shown the feasibility of that. A one-two punch—diagnostic, then therapeutic—is easily imaginable with a Neuralink mesh implant. Things are moving very quickly.
Imaging in Psychiatry
Briefly, there are three primary imaging exams: MRI, CT, and PET scans. MRIs can be functional or structural, with or without contrast. CT scans can also identify structural abnormalities. PET scans look at radioactive tracers related to metabolism or bound to specific proteins such as amyloid or tau.
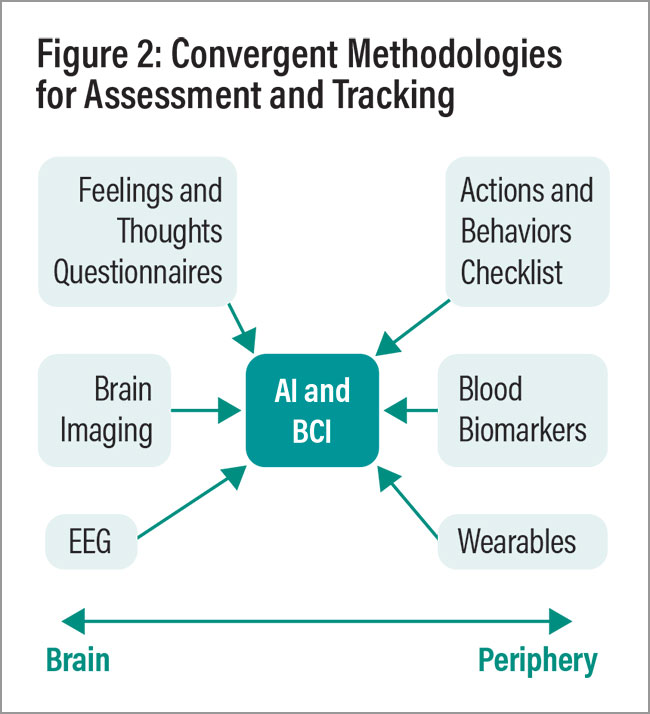
The use of brain imaging in clinical practice for psychiatry has lagged behind its use in neurology and for neurodegenerative disorders. It is possible that by bootstrapping imaging with other modalities, cross-validation can be obtained, delineating useful and predictive findings (Figure 2), including subtypes of different psychiatric disorders (Tozzi, et al., 2024).
Digital Tools in Psychiatry
Digital approaches can be useful, whether involving apps that actively or passively collect information from patients or wearables that track peripheral behavioral measures such as HRV, steps, and sleep. They provide information that is quantitative—and, in the case of wearables, objective. Again, integration and cross-validation with other measures may be helpful.
AI and Psychiatry: Quo Vadis
Good clinical care is based on data and pattern-recognition skills on the diagnostic side, as well as proper decision making and matching on the treatment side. AI arguably can perform better than human clinicians on both counts. For now, two issues need to be resolved: avoiding errors and made-up information (ironically, known as “hallucinations”).
In other words, human supervision and integration remain essential. In the (near) future, after these bugs have been worked out, a lingering issue may or may not be the issue of trust: a suffering patient trusting a non-human entity with their well-being.
The Road Ahead
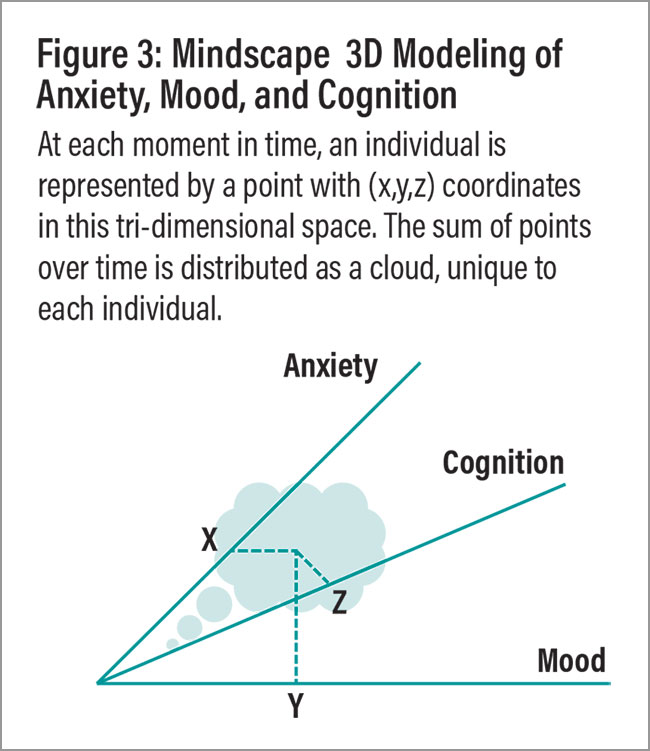
If we were to rebuild psychiatry from first principles, how would it look? An important first step would be to simplify the requirements of diagnosis while making them more precise. One way to do that would be by taking a dimensional approach. For example, DSM/mental health could be reduced to three principal dimensions: anxiety, mood, and cognition. (A fourth dimension could be the environmental effects of trauma.) Together they would create a “mental landscape”/Mindscape model (Figure 3) (Niculescu, et al., 2010), with measurements of anxiety, mood, and cognition leading to (x,y,z) coordinates for each person at a moment in time—a good heuristic step in the direction of personalization.
The future is bright for 21st-century psychiatry. We should develop familiarity with, and start to integrate into actual clinical practice, some or all of the methodologies described above, to deliver care that is precise, personalized, preventive, and participatory. ■