Celiac disease (CD) is an autoimmune disorder that affects genetically susceptible individuals, rendering them unable to digest gluten found in wheat and related grains such as rye and barley. Recent trends indicate a growing prevalence of CD among both pediatric and adult populations (1.4%), likely due to improved diagnostic methods and targeted screening efforts (
1,
2,
3,
4,
5). While the classic CD symptoms include duodenal villous atrophy, malabsorption, failure to thrive, and diarrhea (
6,
7). However, non‐classical manifestations have increasingly come to light. Some patients only discover they have CD through screening investigations prompted by high‐risk factors, such as abdominal pain, altered bowel habits like constipation, anemia, short stature, and other symptoms (
8). Adult‐onset non‐classical CD can also manifest alongside comorbid conditions, including type 1 diabetes mellitus, cancers, skin disorders, gynecologic problems, and neuropsychiatric illnesses (
9). In the 21st century, the pooled incidence of CD among females was 17.4 per 100,000 person‐years, compared with 7.8 among males (
10).
The primary treatment for CD is a lifelong commitment to a GFD (
11,
12). While some suggest that adhering to a GFD can improve gastrointestinal symptoms and potentially alleviate psychiatric disorders (
5). There is an opposing view that GFD may negatively impact the quality of life for CD patients and increase the risk of psychiatric comorbidity (
5,
13). Numerous studies have explored the link between CD and psychiatric disorders (
14,
15,
16,
17). We can generally divide their theories into two schools; specific and non‐specific mechanisms (
17). Specific mechanisms involve biological processes that point to overlapping pathologies, such as the proposed “gut‐brain” relationship (
15,
17,
18). While non‐specific mechanisms encompass the social and emotional consequences of a CD diagnosis (
13).
This systematic review and meta‐analysis aimed to investigate the measures of associations between CD and psychiatric disorders, specifically anxiety and depression, and explore the relationship between adherence to a GFD and the psychiatric aspects of the disease.
METHODS
Search Strategy
A systemic search was carried out to find relevant articles. We searched the following databases: PubMed, Scopus, and Web of Science. The search was carried out from inception until May 23, 2023, using the following search terms: anxiety, depression, CD, and gluten. No filters were applied, and reference lists of included papers were searched to identify further relevant papers that were not identified during the search.
Study Screening and Selection
The following inclusion criteria were developed: Primary research articles (either observational or experimental) that include participants with a CD diagnosis ‐confirmed either serologically, with anti‐endomysial antibodies or anti‐tissue transglutaminase antibodies, or with duodenum biopsy, whether on a GFD or not,—who have depression or anxiety symptoms identified through self‐report or clinician‐administered scales. First, articles were screened by title and abstract by four independent authors in a blinded fashion. Articles that did not meet the inclusion criteria were excluded, and any differences were settled by the first author. Full texts of articles that met the inclusion criteria were retrieved and screened by two independent reviewers and conflicts were settled by the first author.
Quality Assessment
We assessed the quality of case‐control studies using New Castle Ottawa scale (NOS) (
19), studies with a score of 7–9 were of high quality, 4–6 of moderate quality and 1–3 of low quality. Quality assessment of cross‐sectional and cohort studies was assessed using National Institute of Health (NIH) tool (
20), studies with a score of more than 8 are considered of good quality, 5–8 of fair quality and less than 5 are of poor quality.
Data Extraction and Statistical Analysis
Data were extracted from each study by four independent reviewers, with conflicts settled by the first author. Extracted data included: study design, country of study, the study population, sample size and characteristics (age and sex), and the number of subjects suffering from anxiety or depression. Furthermore, we collected the reported scores of The State‐Trait Anxiety Inventory (STAI), Zung Self‐Rating Depression Scale (SDS), Hospital Anxiety and Depression Scale (HADS), Children's Depression Inventory (CDI), and other scales, for both celiac patients and controls.
Statistical Analysis
We conducted the meta‐analysis by pooling the results using Review Manager V. 5.4 software. Random effect model was utilized in pooling with a p‐value of 0.05 and a confidence level of 95%. The analysis for dichotomous variables was done using event and total to calculate the odds ratio, while that of continuous variables was done using mean difference. Heterogeneity between studies was assessed using I2 statistical test. A value of p < 0.05 was statistically significant. We used Open meta‐analyst software for sensitivity analysis using leave‐one‐out method and to calculate the overall mean of different scales.
DISCUSSION
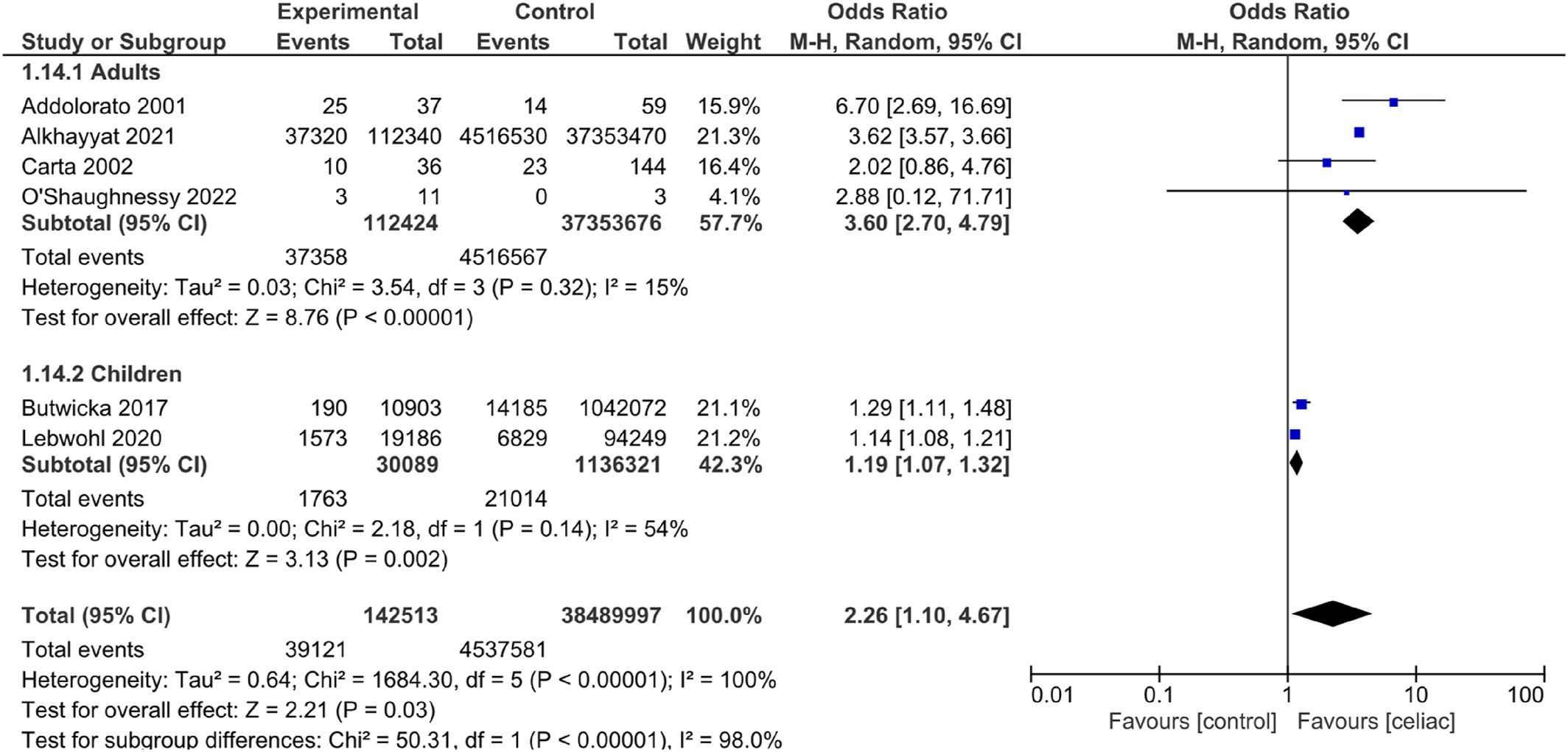
Our study showed the increased odds of anxiety and depression among CD patients. This was assessed using different anxiety and depression scores such as STAI‐Y1, STAI‐Y2, HADS for anxiety and depression, CDI, and Zung‐SDS. It was found that anxiety measured by STAI‐Y1 and STAI‐Y2 was reduced after 1 year of GFD.
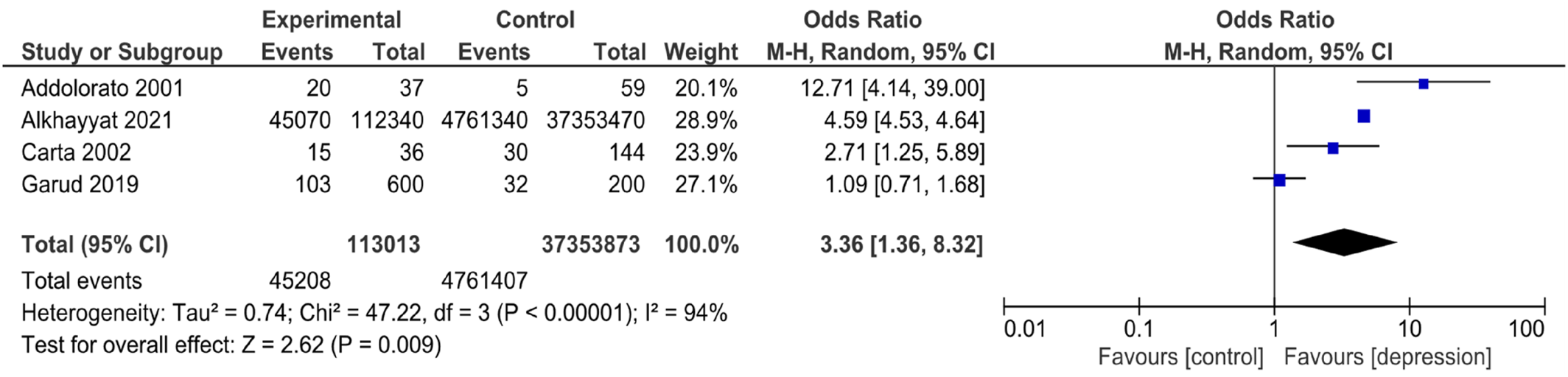
The literature has reported associations between CD and a wide spectrum of psychiatric disorders. According to certain studies (
2,
25), depression was a common comorbidity among CD patients, however, other studies found no differences between CD patients and the general population (
35,
39). It is believed that persistent symptoms like pain or diarrhea commonly occur before the onset of depression symptoms. It is also reported that the severity of depression symptoms is associated with the severity of Gastrointestinal symptoms (
40,
41,
42). A GFD has been shown to reduce the symptoms of depression (
43,
44). Other research (
45,
46), however, revealed that depression symptoms persisted, probably as a result of dietary restrictions that harm patients' social contacts and lower their quality of life (
22). Furthermore, not adhering to a GFD may result in or exacerbate recurrent depression (
47).
The purpose of the study was to assess the occurrence of anxiety and depression in CD patients and if there is an effect of GFD. There are several findings in the study that confirm this association in both adults and children. Our results reveal that there is an increased odds of anxiety and depression in patients with CD. This finding is directly in line with previous studies on CD patients (
48,
49,
50). Also, it has been reported that anxiety and depression can decrease the quality of life in CD patients (
51,
52). So, different scales for anxiety and depression (
53,
54) are used as a measure to diagnose trait and state anxiety, and to distinguish them from depressive disorders. We found that both STAI and HADS scales for anxiety had high values, indicating higher odds of anxiety, as reported in previous research that showed an increase in mean anxiety scores in CD patients (
36). In addition, both HADS and Zung‐SDS for depression had high values, indicating higher odds of depression; this is congruent with another study that reported a significant increase in the percentage of depression among CD patients compared to healthy controls (
21). We also performed a single‐arm meta‐analysis on the scales of anxiety and depression in both adults and children with CD, the results showed increased average means in all scales indicating the strong association between these psychological disorders and CD. These resulting values can provide an estimate of the values of these scales in CD patients and can further help diagnose anxiety and depression in CD patients.
The CDI is a specific scale to measure depression in children (
55), our analysis showed high values indicating higher odds of depression in children with CD. This finding is consistent with a previous study that showed significant improvement in depressive symptoms in children with CD (
56). However, another study on CD pediatric patients showed no significant difference in depression compared to healthy controls (
24).
GFD is considered the most reliable and radical treatment for CD symptoms, as it aids good absorption by improving the structural and functional aspects of the intestinal mucosa and preventing complications of CD which may be malignant or non‐malignant (
57,
58). For this reason, we analyzed studies that reported the values of the STAI‐Y1 and STAI‐Y2 scales of patients who were on a GFD for 1 year to deduce if there is an improvement in symptoms; we found that values improved after GFD, which indicates an improvement in symptoms. Previous research (
22) supported these findings regarding anxiety but not depression, as it showed decreased anxiety after 1 year of GFD. It reported an increased prevalence of state anxiety while there was no change in trait anxiety between patients and controls. This indicates that anxiety in CD patients is primarily considered a reactive form, not a personality trait.
Despite the benefits of GFD, on the other hand, a previous study showed that there was a decrease in quality of life in patients on GFD for 10 years, so, it was reported that long‐term GFD is insufficient to improve the symptoms of CD patients (
57).
Although several studies reported the link between CD and psychiatric disorders, the literature is evermore conflicting due to limitations in knowledge and the need for further different types of studies to strongly prove this association (
57,
59). Also, some studies with small sample sizes may make the findings less reliable and less generalizable (
21). The included studies showed significant heterogeneity, this could be due to conflicting literature or reflecting that the subject is still under investigation and there is a need for more future research to be carried out.
The observed heterogeneity in our findings regarding the association between CD and psychiatric disorders such as anxiety and depression may be influenced by cultural factors. Cultural norms and values can significantly impact the prevalence, expression, and reporting of these psychiatric symptoms. These differences not only encompass the willingness of individuals to report symptoms and seek help but also extend to diagnostic practices and societal acceptance of both CD and mental health conditions. Additionally, the cultural context can affect dietary habits and the availability of gluten‐free options, influencing the ease with which individuals adhere to a GFD and its subsequent impact on mental health.
The study had some limitations, including heterogeneity in the included studies, as the included studies exhibited significant heterogeneity, possibly due to the difference in populations and the assessment scales. However, it was resolved by subgrouping and leave‐one‐out analysis. We conducted a “leave‐one‐out” sensitivity analysis to assess the robustness of our meta‐analytic findings on the association between CD and anxiety/depression. This sensitivity test, contrary to suggesting correlations, was primarily employed to determine the impact of excluding individual studies on the overall results. The “leave‐one‐out” sensitivity analysis ensured the reliability of our findings by identifying the influence of individual studies on the overall results, highlighting the impact of study‐specific factors such as design and population characteristics on the observed association between CD and psychiatric disorders. By conducting this leave‐one‐out, heterogeneity was resolved by the removal of the individual studies that caused this heterogeneity. Some studies had small sample sizes, which could potentially impact the reliability and generalizability of their findings. Long‐term effects of GFD: while our analysis suggests an improvement in anxiety symptoms after 1 year of a GFD, it's worth noting that only two studies investigated this important concern and after only 1 year, emphasizing the need for further investigation into the long‐term effects of GFD on CD patients. Therefore, further longitudinal studies with large sample sizes are recommended to investigate the anxiety and depression in CD patients and the role of GFD in those patients.
In conclusion, the CD is associated with high odds of anxiety and depression in both adults and children. This requires the monitoring of these symptoms and providing mental health care for CD patients. However, extended research is required to understand the specific pathophysiological aspects and the long‐term effects of GFD.