Psilocybin and Motor Function: A Triple‐Blind, Dose‐Finding Study in Healthy Participants
Abstract
Background
Methods
Highlights
Background and Rationale
Objectives
Hypothesis
Primary Aims
Exploratory Aims
Trial Design
METHODS: PARTICIPANTS, INTERVENTIONS, AND OUTCOMES
Study Setting
Eligibility Criteria
Inclusion Criteria
Exclusion Criteria
Medical Exclusion Criteria
Psychological Exclusion Criteria
Study Team Eligibility
Recruitment
Consent
Assignment of Interventions
Allocation Sequence
Blinding
| Block 1 (n = 6); max psilocybin dose = 15 mg | |
| 5 mg dose | 1 × 5 mg psilocybin capsule + 2 × placebo capsules |
| 10 mg dose | 2 × 5 mg psilocybin capsule + 1 × placebo capsules |
| 15 mg dose | 3 × 5 mg psilocybin capsule |
| Block 2 (n = 6); max psilocybin dose = 20 mg | |
| 10 mg dose | 2 × 5 mg psilocybin capsule + 2 × placebo capsules |
| 15 mg dose | 3 × 5 mg psilocybin capsule + 1 × placebo capsules |
| 20 mg dose | 4 × 5 mg psilocybin capsule |
Interventions
Intervention Description
Discontinuing or Modifying Allocated Interventions
Concomitant Care
Contraindicated medications
Follow‐Up, Adherence, and Retention
Outcomes
| Domain | Measures | Application and clinical relevance | |
|---|---|---|---|
| Primary outcomes | Movement tasks | De Morton Mobility Index (DEMMI) (15) | 15‐Item unidimensional instrument that assesses mobility from bed‐bound to independent mobility including balance, gait, seating, and strength. Eleven items are dichotomous (scored 0 or 1), and four items have three response options (scored 0, 1, or 2) based on ability and time to completion. It has high reliability (Pearson's r = 0.94), and high convergent and discriminant validity |
| Functional Movement Exploration | FND extension module developed by our team, based on the Physio4FMD randomized controlled trial in FND participants (16), to assess additional movements including standing up from a chair, weight shifting, balance, and coordination. Items are dichotomous (scored 0 or 1) based on ability, with additional scores for duration where relevant | ||
| Safety | Vital signs and adverse events form | Safety will be assessed throughout the study via monitoring vital signs and recording treatment‐emergent adverse events in the adverse events form. These will be classified based on the type, number, severity, relatedness to the study drug, and whether the event constitutes a serious adverse event or significant safety issue | |
| Exploratory outcomes | Movement tasks | Action Research Arm Test (ARAT) (18) | 19‐Item instrument assessing upper limb coordination, dexterity, and functioning. It has shown excellent inter‐rater and intra‐rater reliability and validity in this setting |
| Box and Block Test (BBT) (19)—original and modified | Original—movement of blocks from one box to another over 60 s, assessing unilateral gross manual dexterity. It has shown high test‐retest validity from normative data in adults without disability | ||
| Modified—places a shield between the participant's vision and their hands, with access to a mirror to view their movements via reflection. This modulates attentional mechanisms of movement—an important feature of motor FND management | |||
| Digit Symbol Substitution Test (DSST) (20) | Symbols are written according to numbers presented via a key within 90 s. This recruits a range of cognitive operations including motor speed, attention, and visuoperceptual functions, and has shown good reliability | ||
| Reaction Time Ruler Drop Test (RTRDT) (21) | Participants catch a ruler dropped by the examiner from a vertical height. It has shown excellent test‐retest and interrater reliabilities and has shown to be useful in assessing reaction time | ||
| Video footage | Review of video footage of movement tasks to assess psilocybin's effects on movement quality | ||
| Verbal fluency | Phonemic and semantic fluency | Participants name as many words as possible beginning with the same letter (phonemic) or of the same category (semantic) in the space of 1 min. These tests recruit a range of cognitive functions across attention, executive function, memory, and language (22) | |
| Brain activity | Resting‐state fMRI | Changes in resting‐state measures of brain activity 1 week following low‐dose psilocybin | |
| Subjective intensity | 5‐Dimensional Altered States of Consciousness (5D‐ASC) | 94‐Item scale across five dimensions: oceanic boundlessness, dread of ego dissolution, visionary restructualization, auditory alterations, and vigilance reduction (23). Responses are rated on a visual analog scale, from “No, not more than usually” to “Yes, much more than usually” | |
| Ego‐Dissolution Inventory (EDI) | 8‐Item focused assessment of the experience of ego‐dissolution (24). Each item is rated on a visual analog scale from “No, not more than usually,” to “Yes, entirely or completely.” It has shown discriminant and convergent validity and excellent internal consistency | ||
| Qualitative experiences | Qualitative interview | Qualitative interview exploring subjective experiences of psilocybin and completing movement tasks and other assessments during the acute drug effects |
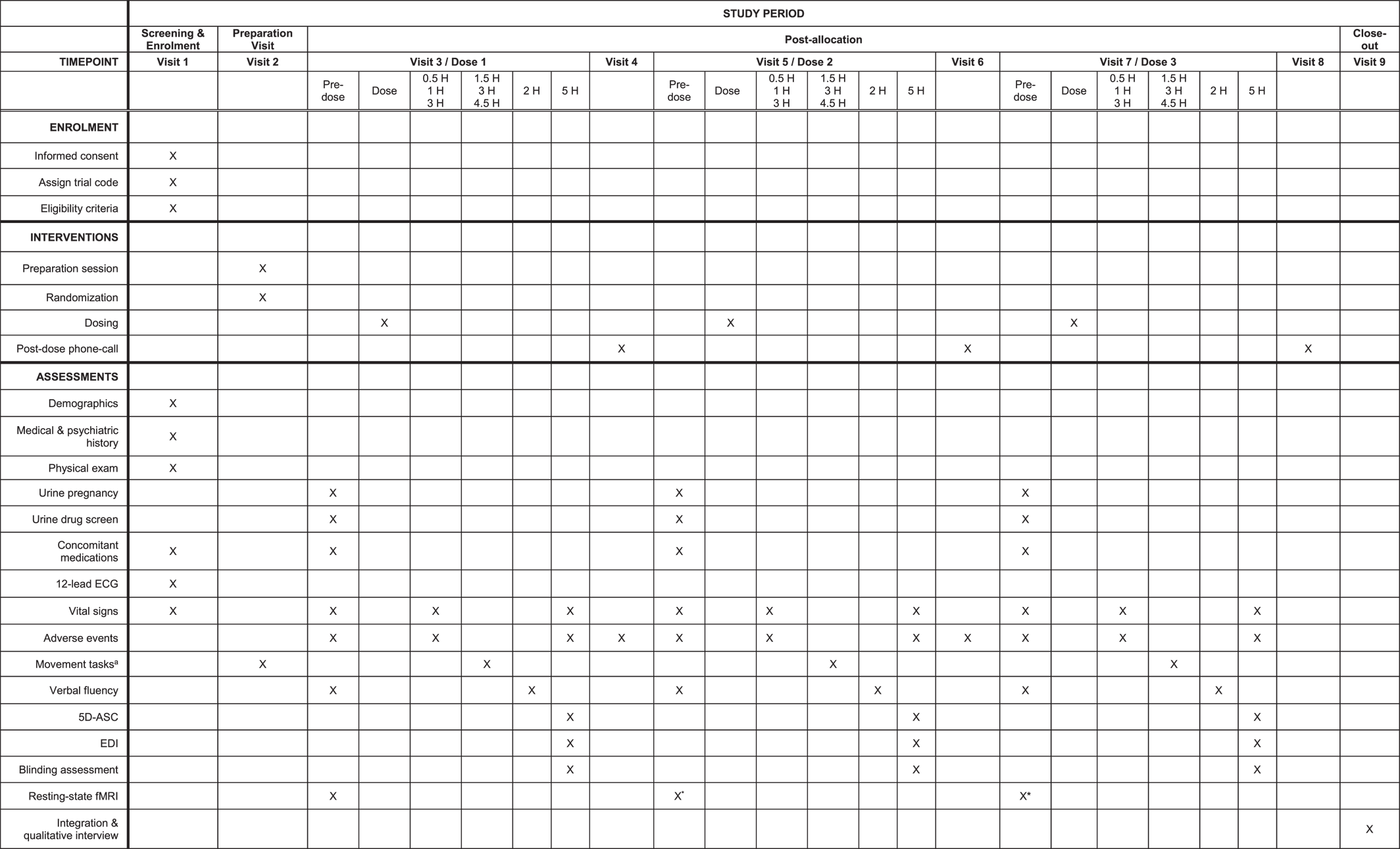
Primary Outcomes
Exploratory Outcomes
Sample Size
DATA COLLECTION, MANAGEMENT, AND ANALYSIS
Data Collection Methods
Data Management
Statistical Methods
MONITORING
ADVERSE EVENT REPORTING AND HARMS
ETHICS
DISSEMINATION PLANS
CLINICAL IMPLICATIONS
Footnotes
REFERENCES
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
