Pharmacologic agents are commonly used to treat pediatric bipolar disorder (
2–
5), even though few are indicated for use by pediatric populations (
6–
8) and most evidence for pediatric use comes from short-term studies of children ages ten and older. To date, the U.S. Food and Drug Administration has granted indications for only five agents for treating acute mania among youths—lithium (ages 12 and older), risperidone, aripiprazole, and quetiapine (ages ten and older), and olanzapine (ages 13 and older) (
9).
However, in recent years there has been a significant shift in the conceptualization and treatment of bipolar disorder among children, and the evidence base for treatment has expanded (
7,
12–
14). For example, the 1997 guidelines emphasized the use of lithium or divalproex as the mainstay of pharmacotherapy and recommended use of antipsychotic medications only after other antimanic agents had been tried (
11). However, as second-generation antipsychotic agents entered the pharmaceutical marketplace, evidence rapidly accumulated for their use in treating bipolar disorder. In response to the growing evidence base, in 2005 and 2007 recommendations for initial pharmacotherapy for children who had been newly diagnosed as having bipolar I disorder were revised (
6,
15). The extent to which current guideline recommendations are followed in clinical practice is unknown.
In addition, although studies have assessed whether patient and provider characteristics are associated with the receipt of guideline-recommended care among children with other psychiatric conditions of childhood, such as attention-deficit hyperactivity disorder (ADHD), depression, and conduct disorder (
16–
20), previous studies have not assessed whether they have any influence on receipt of guideline-recommended care among children with bipolar disorder. Understanding the role that patient and provider characteristics play in the receipt of recommended pharmacotherapy by children with bipolar disorder could provide targets for quality improvement efforts in this population. Specifically, these factors may help to inform future dissemination efforts by determining whether individual and contextual factors are associated with the adoption of guideline recommendations in routine practice (
21).
Given the reported increases in pediatric bipolar diagnoses and the expansion of pharmacologic treatment options over recent years, it is important to understand the quality of current medication prescribing among pediatric patients. The objectives of this study were to identify the extent to which guideline-recommended, first-line treatments are received and patient and provider characteristics associated with receiving guideline-recommended treatment among a retrospective cohort of children with newly diagnosed bipolar I disorder.
Methods
Data source and study design
We conducted a longitudinal, retrospective person-level cohort study of commercial claims data collected by Thomson Reuters MarketScan from January 1, 2005, through December 31, 2007. MarketScan includes deidentified data on clinical utilization and expenditures for inpatient, outpatient, and pharmacy services for approximately 100 privately insured payers within the United States. This study received a waiver from the University of North Carolina at Chapel Hill's Institutional Review Board.
Sample selection
Children ages six to 17 who had at least one inpatient or two outpatient claims for bipolar I disorder manic or mixed episode type (
ICD-9 codes 296.4 or 296.6) (
22–
24) and who were continuously insured for 180 days before and one year after their first observed (index) date of diagnosis were included. Consistent with previous research on pediatric bipolar disorder (
25), we excluded patients with schizophrenia, pervasive developmental disorders, mental retardation, or substance abuse disorders in the 180 days before their bipolar diagnosis. Children with medical conditions that mimic symptoms of mania, such as temporal lobe epilepsy, multiple sclerosis, hyperthyroidism, closed or open head injury, and systemic lupus erythematosus, or conditions that complicate the treatment of bipolar disorder, such as pregnancy, were also excluded (
26,
27). Overall, 1,324 children met these criteria.
To ensure that the sample included only children with new diagnoses for bipolar disorder, we excluded 487 children who filled a prescription for an antipsychotic, anticonvulsant, or lithium during the 180 days before the index bipolar diagnosis. Because we were interested in evaluating the initially prescribed treatments, we excluded another 228 children who had been hospitalized within 60 days before or 45 days after the index bipolar diagnosis because the prescription claims records did not include medications received in the hospital. Finally, 197 patients whose insurance plans did not provide information about medication use were excluded. In total 412 children met all inclusion and exclusion criteria.
Study variables
The primary dependent variable was receipt of guideline-recommended initial pharmacotherapy within 90 days of a child's first bipolar diagnosis. We used the 2005 and 2007 expert-consensus treatment guidelines to define guideline-recommended initial pharmacotherapy (
6,
15). Guideline-recommended pharmacotherapy was defined as having received lithium, divalproex, carbamazepine, olanzapine, quetiapine, risperidone, ziprasidone, or aripiprazole within 90 days of the index diagnosis. Monotherapy with other psychotropic medication or combination pharmacotherapies are considered nonrecommended first-line treatments. Additionally, children who did not receive a recommended medication within 90 days of the initial bipolar diagnosis were considered to have not received guideline-recommended therapy.
Independent variables included patient demographic and clinical factors. Demographic characteristics included patient age at the time of diagnosis and sex. Clinical characteristics included other mental health diagnoses that the child had received within 180 days before the index bipolar diagnosis—ADHD, other disruptive disorders (conduct and oppositional defiant), anxiety disorders (separation anxiety, posttraumatic stress, obsessive-compulsive, generalized anxiety, social phobia, and panic), and depressive disorders (major depressive and dysthymic). Two variables—the presence of inpatient mental health days during the 180 days before and 365 days after the index bipolar diagnosis and the presence of psychosis, indicated by the fifth digit of the
ICD-9 code—were included as measures of disease severity (
28).
Additionally, because use of psychotherapy could indicate care of higher quality when used as an adjunctive treatment to pharmacotherapy or as care of lower quality when used as a substitute for pharmacotherapy, we controlled for the receipt of psychotherapy in the 90 days before or the 30 days after initial treatment (
6,
15). Finally, by using the provider specialty variable available on inpatient and outpatient claims, an indicator was created to identify whether a child received care for bipolar disorder from a general or child psychiatrist on the day of or during the 180 days before the first medication fill date.
Statistical analysis
We calculated the proportion of children receiving recommended care within 90 days of their initial diagnosis and prepared summaries of specific treatment regimens received.
Bivariate relationships between patient and provider characteristics and the likelihood of receiving recommended care were tested by using Fisher's exact tests. Multivariate models were estimated to assess factors associated with receiving recommended care. Log binomial models controlling for other variables were used to determine the effect of each predictor on the likelihood of receiving guideline-recommended care (
29–
31). Risk ratios (RR) and 95% confidence intervals (CI) are presented for all comparisons. We used SAS, version 9.1, for all analyses.
Sensitivity analyses
We also assessed the proportion of children receiving guideline-recommended pharmacotherapy within 180 days and one year of the index bipolar diagnosis to account for delays in the receipt of pharmacotherapy.
Similarly, we considered two alternative definitions of recommended care. First, to account for clinical use of nonrecommended agents for which new evidence has accumulated, we used an expanded definition of guideline-recommended care that included lamotrigine, an anticonvulsant that has been approved for use as maintenance treatment for adults, as an acceptable treatment option for children with bipolar disorder (
15). Second, because treatments prescribed but not utilized by the patient cannot be observed, we restricted analyses to users of psychotropic medication. By restricting the sample in this way, cases in which a patient failed to fill a prescription for a guideline-recommended medication would not be misclassified as examples of nonrecommended treatment.
Next, because evidence suggests that many children with bipolar disorder do not receive antimanic treatment (
32), we assessed patient and provider factors associated with receiving any pharmacotherapy.
Finally, a previous validation of adult bipolar disorder found that billing claims with a bipolar diagnosis submitted by primary care physicians were not supported by chart reviews (
33). To address this concern, we conducted a final sensitivity analysis in which we restricted our sample to patients who received care from a psychiatrist in the 180 days preceding the index diagnosis.
Results
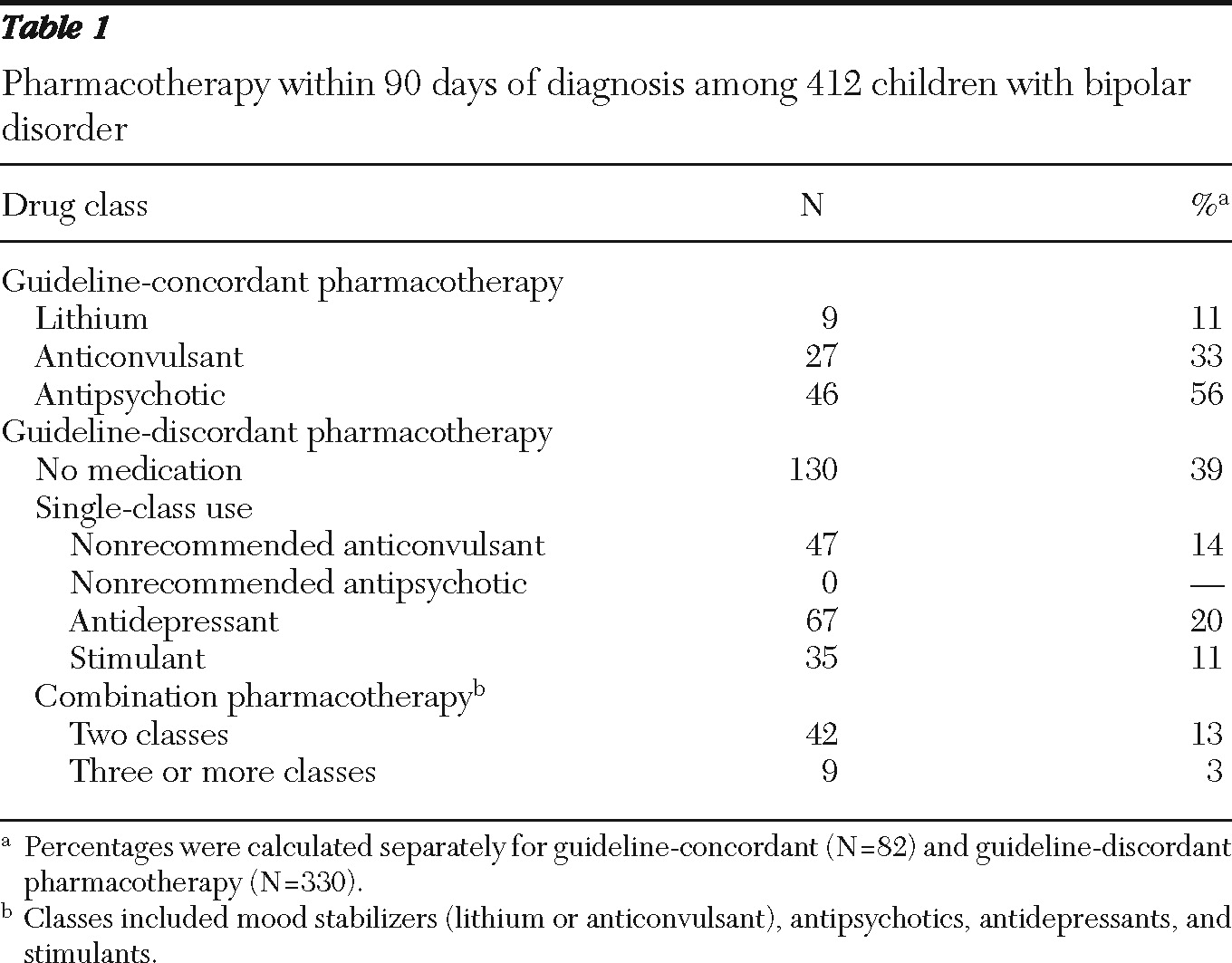
Overall, only 82 of 412 (20%) children with bipolar I disorder received guideline-recommended medications within 90 days of diagnosis. Among the 82 who received guideline-recommended care, 56% received antipsychotics alone, 33% received anticonvulsants alone, and 11% received lithium alone. Among the 330 children who did not receive guideline-recommended care, 39% received no psychotropic medications during the first 90 days (
Table 1).
Of the 282 children receiving any psychotropic medication within 90 days of diagnosis, 200, or nearly 71%, received nonrecommended treatments. Approximately 24% received antidepressant monotherapy, and 18% received combination pharmacotherapy, neither of which is consistent with recommendations.
Results from sensitivity analyses indicated that slightly more children had received recommended medications by six months (N=91, 22%) or one year (N=99, 24%). By one year, 91 of the 412 (22%) children had received no medication, and 222 (54%) had received nonrecommended pharmacotherapy.
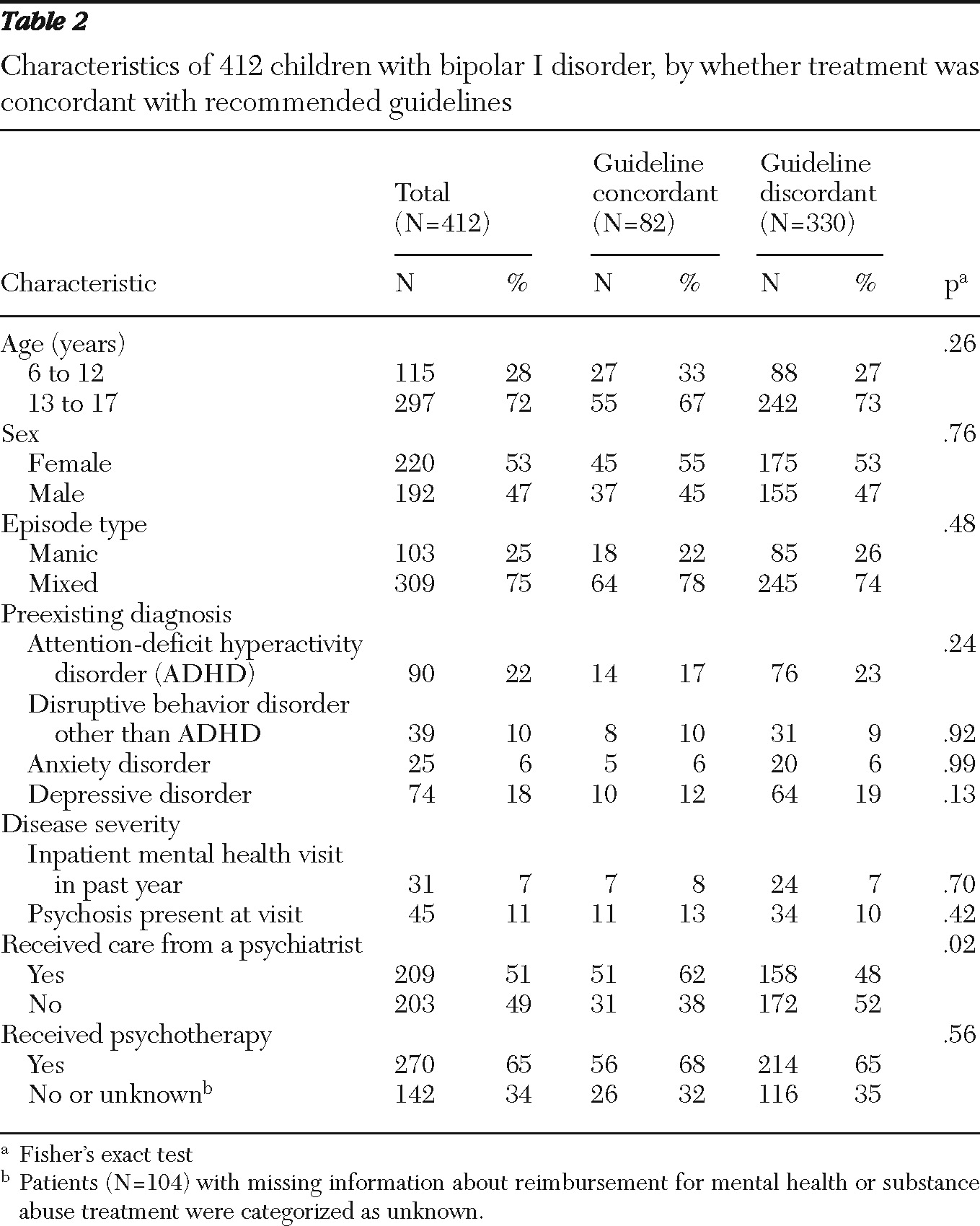
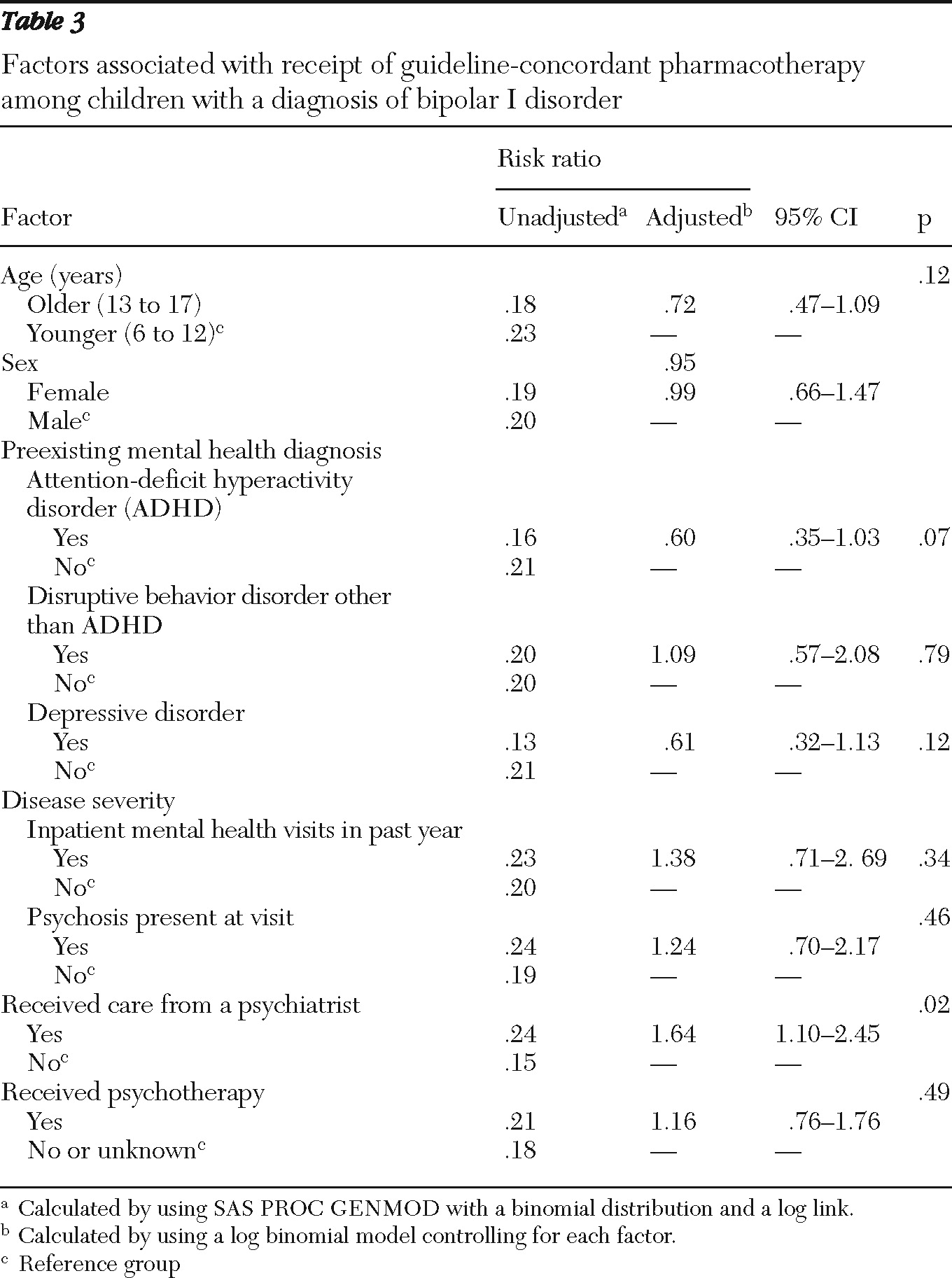
Receiving care from a psychiatrist was the only variable found to be statistically significantly related to receiving guideline-recommended pharmacotherapy by both bivariate analysis (
Table 2) and in the fully adjusted model (adjusted RR=1.64) (
Table 3). However, the likelihood of receiving recommended pharmacotherapy was low regardless of provider type. Only 51 of 209 (24%) children treated by a psychiatrist received guideline-recommended pharmacotherapy, compared with 31 of 203 (15%) children who were not treated by a psychiatrist.
In sensitivity analyses that expanded the definition of recommended care to include lamotrigine, receiving care from a psychiatrist was also found to be related to a higher likelihood of receiving guideline-recommended care (RR=1.85, CI=1.36–2.51). Additionally, when lamotrigine was included as an acceptable treatment option, having received a diagnosis of ADHD (RR=.61, CI=.40–.92) or a depressive disorder (RR=.64, CI=.41–.98) was related to a lower likelihood of receiving guideline-recommended pharmacotherapy.
Sensitivity analyses that excluded patients who did not receive any psychotropic medications also found that preexisting ADHD (RR=.59, CI=.35–.98) or a depressive disorder (RR=.51, CI=.28–.94) were related to a lower likelihood of receiving recommended pharmacotherapy, but receiving care from a psychiatrist was no longer a statistically significant predictor of receiving recommended care. However, children who received care from a general or child psychiatrist were more likely to receive any psychotropic medication than children who were not treated by a psychiatrist (RR=1.13, CI=1.02–1.24).
Finally, sensitivity analyses of patients who received care from a psychiatrist on the day of or during the 180 days before the index diagnosis found that older age (RR=.54, CI=.33–89) and preexisting ADHD (RR=.48, CI=.24–.96) were related to the likelihood of receiving recommended care.
Discussion
Despite the availability of evidence-based treatment guidelines, more than 80% of children with newly diagnosed bipolar I disorder failed to receive recommended first-line pharmacotherapy. The majority of patients in this sample received either no medication or antidepressant monotherapy in the 90 days following their initial diagnosis. Notably, because these children had health insurance, at least one barrier to receiving appropriate treatment, lack of coverage, had been addressed.
Nonuse of antimanic treatments among children with bipolar disorder has been previously reported (
32), especially in pediatric rather than psychiatric settings (
34). Reasons for nonuse of pharmacotherapies may include a provider's discomfort with prescribing medications, concerns related to the medication's side effects, or parents' hesitation to accept a child's bipolar diagnosis and treatment (
15,
35–
37) In fact, our results suggested that there may be initial reluctance to prescribing or filling medications—pharmacologic treatment increased by 28% when the time frame for assessment was expanded from 90 days to one year after diagnosis.
Not only did a large percentage of children receive no pharmacotherapy, many children received antidepressant monotherapy, a treatment strategy that is considered questionable for both children and adults with bipolar disorder (
38). There are two major concerns about treatment of bipolar disorder with antidepressant agents. The first is the potential for causing manic switching (
39), particularly among children experiencing a depressive episode, given that use of antidepressants in the absence of mood stabilizers may increase the risk of developing mania (
40,
41). The second concern is related to fears of triggering suicidal behaviors among children with depressive disorders by treatment with selective serotonin reuptake inhibitors (
42,
43). This concern is especially worrisome because children with bipolar disorder are already at a much greater risk for suicidal behaviors and suicide completion than other children, including children with depression (
44).
Previous studies of guideline concordance have largely focused on assessing the extent to which adults with bipolar I disorder received recommended pharmacotherapy. Such studies have shown wide variation in the percentage of patients who received guideline-recommended pharmacotherapy—among outpatient samples, estimates have ranged from 67% to 84% (
45–
48). Such findings are in stark contrast to the low rates identified by this study and by a previous study of guideline-concordant prescribing among youths (
10).
One potential explanation for the differences in use of evidence-based guidelines for children and adults is uncertainty about the diagnosis among children (
49). The criteria for diagnosing children are not well established, and the use of the bipolar diagnostic label among children with nonclassical symptom presentations is a matter of debate in the field (
15,
50). When children with subsyndromal symptoms receive a diagnosis of bipolar disorder (
2), initial treatment may be delayed while the physician observes symptoms over time. Variations in the application of the bipolar diagnostic label may also explain why few factors consistently predicted receipt of recommended pharmacotherapy.
Receiving care from a psychiatrist was associated with a higher likelihood of receiving guideline-recommended care in the primary analysis and when the analysis was expanded to include lamotrigine as an acceptable treatment. Analyses of predictors of psychotropic medication use also suggested that children who received care from a psychiatrist were more likely to receive psychotropic medications than children who did not receive care from a psychiatrist. However, when the patient population was restricted to those who received pharmacotherapy, the difference was no longer statistically significant, suggesting that children who received medications were equally likely to receive guideline-recommended treatment independent of provider type.
Although having a previous mental health diagnosis was not associated with receiving recommended pharmacotherapy in the primary analysis, sensitivity analyses suggested that previous mental health diagnoses may play an important role. In particular, among children who received medications, those with preexisting ADHD or depressive diagnoses were less likely to receive guideline-recommended pharmacotherapy. The high levels of antidepressant and stimulant use in the sample overall may be related to ongoing treatment of those disorders. However, guidelines specifically recommend that after a bipolar diagnosis, ongoing therapies be discontinued and comorbidities reconsidered only after the bipolar disorder is stabilized (
6). A similar relationship between the preexistence of mental health diagnoses and guideline-discordant treatment of bipolar disorder has been described among adult Medicaid patients (
22).
Although we were unable to detect a consistent relationship between the severity of a child's bipolar disorder and his or her likelihood of receiving recommended care, these factors may be important. For example, patients who are treated in outpatient settings or by practitioners that do not specialize in child psychiatry may be less severely ill than those treated in specialty clinics (
34). More detailed information about the patient's clinical characteristics might clarify this relationship.
Our study had several limitations. First, use of administrative claims data to identify patients with bipolar I disorder may lead to misclassification bias. To increase the reliability of the diagnosis, we included only children with more than one claim for outpatient treatment of bipolar disorder or a claim for inpatient treatment of bipolar disorder (
51). Moreover, we restricted our sample to patients with
ICD-9 codes for manic or mixed episode types. Intuitively, requiring this higher level of episode specification should increase the specificity of the diagnosis.
Additionally, the sensitivity analyses that restricted the sample to children who received care from a psychiatrist in the 180 days prior to the diagnosis presumably limited the sample to children who were correctly classified as having bipolar disorder. Nonetheless, the analyses also found that fewer than one in four received guideline-recommended initial pharmacotherapy.
Second, we lacked data on patients' use of medication other than what was indicated by insurance claims, for example, medication samples or cash purchases, and we could not capture information about pharmacotherapy or psychotherapy that was prescribed for the patient but not utilized. Finally, restricting our sample to children who had been given an ICD-9 diagnosis with codes indicating a manic or mixed episode limited the sample size and reduced the statistical power of the analyses. However, the guidelines' recommendations were specifically tailored to children with bipolar disorder who present with a manic or mixed episode. Therefore, the smaller sample size increased the internal validity of the analyses. Because the focus of this work was to identify potential associations between use of guideline-concordant treatment and characteristics of patients and clinicians, rather than to explicitly test a hypothesis, accepting reduced statistical power to improve internal validity seemed to be an appropriate trade-off.