For the past decade, federal agencies and private foundations have sought to improve the quality of substance abuse treatment by promoting the implementation of evidence-based treatment practices (
1–
5). Concurrently, the number of pharmacotherapies has increased to include buprenorphine, acamprosate, and extended-release injectable naltrexone. Along with disulfiram, tablet naltrexone, and methadone, they constitute the medications currently approved by the U.S. Food and Drug Administration (FDA) for treating opioid and alcohol use disorders.
Although these medications are available, relatively few specialty treatment organizations besides opioid treatment programs dispensing methadone prescribe these medications. Adoption is notably low by programs that primarily rely on governmental sources of funding (
6–
9). These publicly funded organizations, which include government-owned programs and nonprofits that contract with governmental entities, deliver the majority of treatment in the United States (
10–
12).
Most studies of adoption of medications for substance abuse have focused on intraorganizational factors and used diffusion theory (
13) to explain the relationships between pharmacotherapy adoption and organizational resources (
14–
16). Certain resources, particularly access to medical staff, have been repeatedly linked to medication adoption (
7–
9,
17–
19). Nonadopting programs frequently cite lack of access to physicians as a highly significant barrier (
20). However, rates of medication adoption continue to be less than 50% even in programs with physicians (
21).
The role of the external environment, particularly state policies and priorities, has been less frequently examined. Sociological theories suggest environmental contexts may influence organizational decision making (
22–
24). Resource dependence theory emphasizes how decisions about innovations reflect attempts to adapt to the social networks in which organizations are embedded (
25,
26). Institutional theory contends that organizations may adopt innovations to enhance their legitimacy with key stakeholders (
27).
For publicly funded substance abuse treatment organizations, state governments represent important network relationships because states are major funders of treatment services (
28). Treatment organizations may financially benefit from government contracts to provide services (
29). States determine whether services are covered by Medicaid (
30), and policies providing financial support for pharmacotherapy vary among tates (
29,
31).
In addition to establishing funding policies, state substance abuse authorities, also known as Single State Agencies (SSAs), may demonstrate normative support for innovations by offering training and disseminating information about implementation (
32). Such activities send signals about the value of an innovation and imply that adoption may enhance programs' institutional legitimacy.
Research on the relationships between state policies and medication adoption is limited. Interviews with representatives of SSAs suggest that many view pharmacotherapy as a state priority but that funding for implementation is often lacking (
33). Two studies have integrated organizational data about medications with measures of state policies constructed from external sources. Heinrich and Hill (
34) merged facility-level data about naltrexone adoption with state-level measures of policy derived from secondary sources. Indicators of restrictiveness in states' Medicaid policies were negatively associated with naltrexone adoption. Similarly, Ducharme and Abraham (
35) integrated facility-level data on buprenorphine adoption with interview data from state authorities. Buprenorphine adoption was significantly greater if the state's Medicaid formulary included this medication.
An alternative approach to measuring the state policy environment is to consider the perspective of treatment programs. To some extent, state policies are meaningful only if they are effectively communicated to treatment programs. Programs unaware of state priorities regarding evidence-based practices may be less likely to make decisions that align their programs with the state policy environment. Resource dependence and institutional theory suggest that programs that are aware of supportive state policies are more likely to adopt those innovations.
Using data collected from publicly funded treatment programs, this study evaluated two hypotheses related to the adoption of medications for treating substance abuse and dependence. The first hypothesis was that treatment programs' perceptions about their SSA's support for medications are positively associated with the odds of adopting at least one medication. Second, we hypothesized that programs' awareness of medication-supportive state funding policies is positively associated with medication adoption. We focused on adoption of any medications because research has shown that adoption of one medication is associated with adoption of others (
8,
36).
Methods
Sample
The study drew upon a previously established, nationally representative U.S. sample of 318 publicly funded substance abuse treatment centers from the National Treatment Center Study. The sample, originally recruited in 2004–2006, was constructed by using a two-stage design that randomly selected U.S. counties from ten population-based strata and then randomly selected treatment organizations within those counties. To be eligible, treatment organizations were required to be open to the public and offer a minimum level of care at least equivalent to structured outpatient treatment. They were also required to have received at least half of their past year's revenues from government block grants or contracts or to have derived at least half of their patients' expected source of primary payment from allocated public funds other than public insurance, such as block grants or contracts. Opioid treatment programs that exclusively dispensed methadone without offering other levels of care were not eligible. Full details of the sampling procedure have been published elsewhere (
20).
Data collection
Data collection occurred between August 2009 and June 2010. Trained interviewers contacted 318 programs by telephone to ascertain whether they still delivered substance abuse treatment. A total of 27 programs (8%) had ceased operations completely or no longer offered substance abuse treatment. The remaining 291 programs were mailed a packet containing a study description letter, two informed-consent forms, the survey, and a postage-paid envelope. Programs that had not responded after six weeks were mailed a second packet. Finally, interviewers contacted nonresponding programs by telephone. Administrators who provided verbal informed consent were interviewed by using the same survey that had been mailed. Participating programs received $50. The research design was approved by the institutional review boards of the University of Georgia and the University of Kentucky.
Data were obtained from 250 administrators of the 291 programs contacted (86% response rate). Nine (3%) administrators declined to participate, and interviews could not be scheduled with 32 (11%) administrators despite repeated attempts. We used data from the 2004–2006 interviews to compare participating programs (N=250) with those that had closed (N=27) and those that did not participate (N=41) on a set of organizational characteristics (
20). Bivariate multinomial logistic regression models indicated that nonparticipating and closed programs did not differ from participating programs on any organizational characteristics except government ownership. The odds of program closure, relative to study participation, were significantly greater among government-owned programs than nongovernmental programs (relative risk ratio=3.28, 95% confidence interval=1.44–7.46; p<.01).
Measures
The survey measured current use of medications, perceptions of the state policy environment, and organizational characteristics. The dependent variable of any medication adoption was constructed from two items. First, administrators were asked whether the program prescribed any medications for the treatment of substance use disorders or psychiatric conditions. If the administrator responded affirmatively, she or he was asked whether the program prescribed only psychiatric medications, prescribed medications only for substance abuse or dependence, or prescribed medications for both conditions. Programs whose practices fell into one of the latter two groups were considered adopters and were coded 1. Programs prescribing only psychiatric medications or no medications were considered nonadopters and coded 0.
Two measures of the state policy environment were constructed. Administrators were asked to rate their agreement with three items, listed in
Table 1, about the SSA's support for medications on a scale of 0, strongly disagree, to 4, strongly agree. Higher scores indicate greater SSA support. These items were combined into a mean score (Cronbach's
α=.78). Awareness of medication-supportive state funding policies was based on two items. For the first item, administrators were asked, “Based on your knowledge, does your state's Medicaid program include any addiction treatment medications on its formulary?” Response options were yes, no, and don't know. Given that state policies are meaningful only if they are recognized by treatment programs, we collapsed the no and don't know responses into a single group.
For the second item, administrators were asked, “Can treatment providers with state contracts to provide addiction treatment services use those state contract funds to pay for the purchase of medications?” Response options were the same as for the first question, and no and don't know responses were collapsed into a single group. We created a typology of four mutually exclusive categories—awareness of both policies, awareness of only a supportive Medicaid policy, awareness of only a supportive state contract funding policy, and awareness of neither policy.
Organizational characteristics included government ownership, location within a health care setting, accreditation by an external organization, availability of medically supervised detoxification, and levels of care. The number of physicians on staff, number of physicians on contract, and number of nurses employed by the organization were measured.
Possible scores on 12-step treatment orientation range from 0, strongly inconsistent, to 4, strongly consistent, with greater scores indicating a stronger orientation toward a 12-step treatment model. Averaging the three items into a mean score (Cronbach's
α=.78) resulted in a measure of consistency between the program's philosophy and a 12-step orientation to treatment (
37).
Although treatment programs were the unit of analysis, we collected basic demographic information about the survey respondents.
Analysis
Descriptive statistics were calculated for the study variables. Given the dichotomous nature of the dependent variable, logistic regression was used to estimate the model of medication adoption (
38). Listwise deletion, or elimination of cases with missing data on the covariates, would have resulted in the loss of 11% of cases, so we used multiple imputation to address covariates with missing data (
39). To be conservative, we excluded two cases with missing data for the dependent variable (
40). Missing values were imputed using “ice” in Stata 11.1 (
41,
42), yielding 20 imputed data sets that were based on the variables in the model. This multiple imputation by chained equations approach is superior to other imputation procedures (
43). We used the “mi estimate” command during model estimation to produce a single set of results that pooled estimates from each of the data sets (
44,
45).
Results
In this sample of 250 publicly funded substance abuse treatment organizations, 91 (37%) programs prescribed at least one of the six FDA-approved medications. Buprenorphine was the most widely adopted medication, although it was prescribed by less than 25% of programs. Descriptive statistics of the programs are presented in
Table 1. About half of respondents (N=134, 54%) were women, and most (N=182, 74%) were white. Only 32 (13%) of respondents reported being African American or black, and 18 (7%) identified as Hispanic or Latino. A majority (N=173, 70%) held at least a master's-level degree, and the average age was 50.8±10.0 years.
Administrators' perceptions about the supportiveness of the state policy environment were mixed. The mean±SD level of perceived SSA support for medications (2.3±.9) was slightly above the scale's midpoint. About one-third (37%) of programs indicated that the state's Medicaid formulary included at least one medication. Less than 30% reported that state contract funding could be used to purchase medications. Substantial percentages of programs were unaware of their states' medication-related funding policies.
At the bivariate level, the state policy environment was associated with medication adoption. Adopters perceived significantly greater SSA support for medications than nonadopters (2.6±.9 versus 2.1±.9, respectively; t=−3.58, df=235, p<.001). A chi square test of the awareness of supportive funding policies and medication adoption revealed varying rates of adoption across the four categories (χ2=52.8, df=3, p<.001). Adoption was quite low in the 128 programs unaware of either policy; only 19% (N=24) were medication adopters. Of the 46 programs aware of a supportive Medicaid policy, 46% (N=21) were adopters, and of the 28 programs aware of a supportive state contract policy 36% (N=10) were adopters. Adoption was highest (79%) in the 43 programs aware of both policies (N=34).
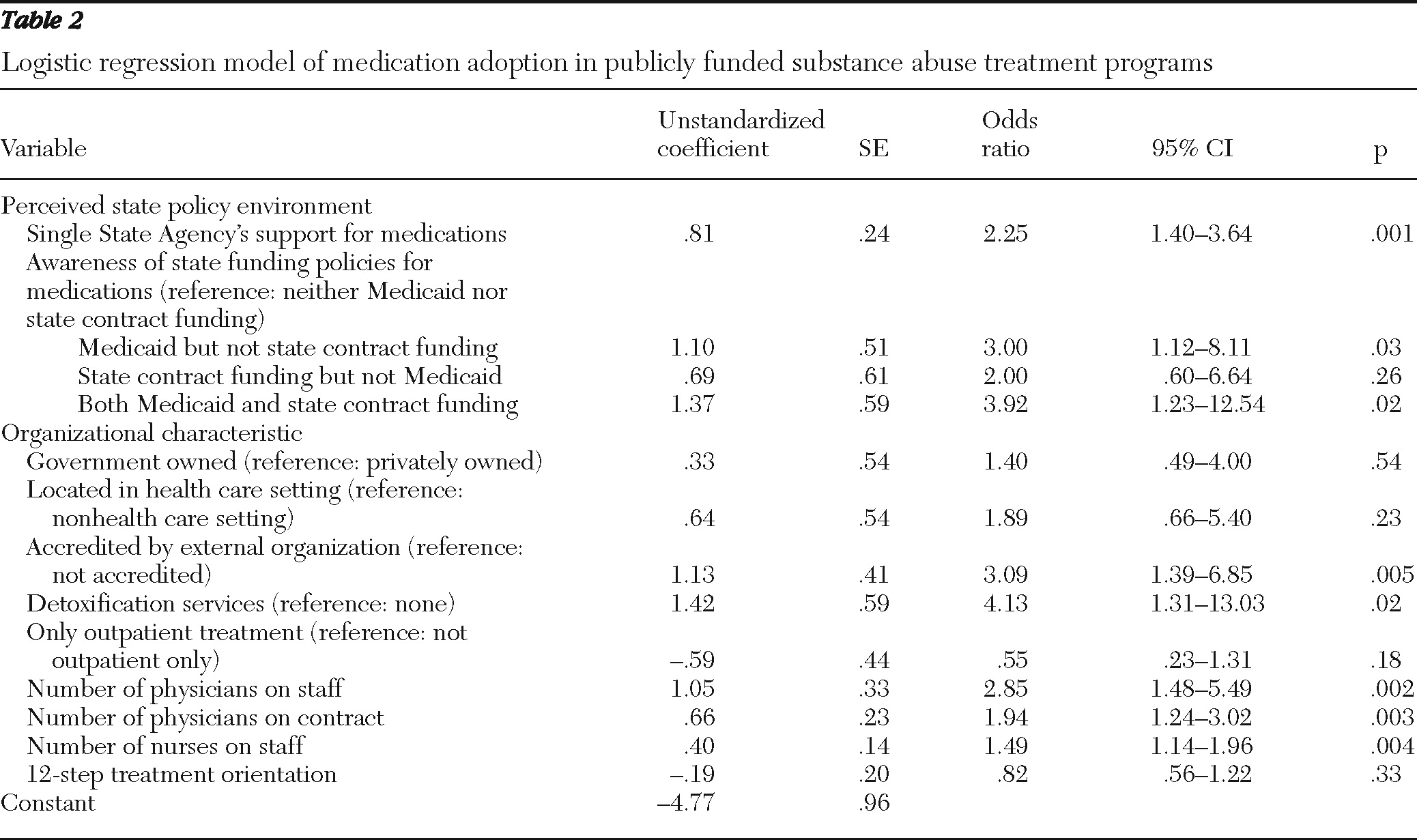
The multivariate logistic regression model of medication adoption appears in
Table 2. Consistent with our first hypothesis, there was a positive association between perceived SSA support and medication adoption. Two of the three comparisons within the measure of awareness of medication-supportive state funding policies were significant, providing partial support for the second hypothesis. The odds of adoption were about three times greater for programs aware of a Medicaid supportive policy than for programs aware of neither a Medicaid nor a state-contract funding policy. The odds of medication adoption were nearly four times greater for programs aware of both policies than for those aware of neither Medicaid nor state-contract funding policies.
The difference in medication adoption between programs aware of a supportive state-contract funding policy and programs aware of neither policy was not significant. Finally, medication adoption was associated with accreditation; the availability of detoxification services; and the number of physicians on staff, physicians on contract, and nurses on staff.
Programs offering methadone are subject to significant regulation, so additional analyses considered whether such programs influenced these results. Exclusion of methadone-offering programs (N=22) had little impact on the association between perceived SSA support and medication adoption. Medication adoption continued to be more likely in programs aware of a Medicaid-supportive policy (p<.01). The association between awareness of both policies and medication adoption approached significance (p=.053). There was some evidence that perceived SSA support may play a mediating role—a positive association was found between adoption and awareness of both policies (p<.05) when SSA support was not entered into the model of medication adoption. In addition, perceived SSA support was positively associated with the likelihood of awareness of both policies (p<.01). [Results of analyses that excluded the methadone-offering programs are available in an online appendix to this report at
ps.psychiatryonline.org.]
Discussion
This study of publicly funded substance abuse treatment programs found that only 37% prescribed at least one medication for treating substance use disorders in 2009–2010. Although this rate was modest, it was about 14 percentage points greater than the adoption rate by the sample in 2004–2006 (
20), an indication of some expansion in the availability of pharmacotherapy.
These findings contribute to the small but growing body of research on state policies and medication adoption. Consistent with resource dependence theory (
24,
25) and institutional theory (
27), we found support for two hypotheses. First, the odds of adoption were greater in programs that reported awareness of two supportive state funding policies, namely, that medications were included in the Medicaid formulary and that state funding policies allowed programs to purchase medications with state contract dollars. Notably, awareness of a policy that allowed the purchase of medications in the absence of awareness of a Medicaid supportive policy was not associated with medication adoption after adjustment for other organizational characteristics and supportiveness of the SSA.
The findings are consistent with other studies highlighting the importance of Medicaid formularies in facilitating the adoption of medications (
34,
35). In addition, we found that efforts by SSAs to actively promote medications were positively associated with the likelihood of medication adoption.
Whereas aligning state funding policies to support medications may promote adoption, the mere presence of policies may be insufficient. Effective dissemination of these policies to treatment programs is critical because a substantial proportion of programs did not know whether their states had implemented medication-supportive funding policies. As long noted by sociologists, reality is socially constructed (
46), and so the existence of supportive state policies is unlikely to be a sufficient driver of innovation adoption if it is not coupled with adequate dissemination.
Our findings also provide empirical support for the strategies proposed by the National Quality Forum to increase the adoption of evidence-based practices (
4). The Forum suggested that financing, regulations and accreditation, training, and infrastructure development may be vital strategies. Although we did not measure infrastructure development, our model supported the other three strategies by the association of awareness of supportive funding policies, provision by SSAs of training and information, and accreditation with medication adoption.
Recent demonstration projects seeking to increase the adoption and implementation of evidence-based practices in substance abuse and mental health treatment also point to the importance of the state policy environment. The Robert Wood Johnson Foundation's Advancing Recovery Initiative partnered state authorities and treatment providers to promote implementation of evidence-based practices (
3,
47). In states focusing on medications, funding policies were identified as critical barriers to implementation and were addressed through redirecting existing state funds to pay for medications, amending state contracts to include medications, and changing states' Medicaid formularies (
48).
Similarly, the experiences of mental health agencies in an evidence-based practices project highlighted the centrality of state mental health authorities in the implementation process (
49,
50). Specifically, greater implementation was accomplished when state mental health authorities clearly communicated how funding policies could be used, offered ongoing trainings, and communicated normative support for implementation (
51).
Several limitations of this study should be noted. First, this sample is representative only of the publicly funded treatment sector in the United States. Although such programs are the largest sector, these findings may not generalize to other sectors, such as privately funded treatment organizations, programs in the Department of Veterans Affairs system, and programs that exclusively dispense methadone. Second, the cross-sectional design and reliance on program administrators' reports limit our ability to establish causality and may have resulted in social desirability or recall bias.
In addition, our measures of the state policy environment were limited. There may be other state policies that serve as barriers and facilitators to medication adoption that we did not measure. Because we did not collect data directly from SSAs to measure state policies, there may be error in programs' awareness of medication-supportive policies. However, data from a state-level report from 2008 allowed us to examine the inclusion of buprenorphine and naltrexone on Medicaid formularies for 83 of the 91 programs reporting that at least one medication was included in the Medicaid formulary (
52). A total of 75 programs (90%) were located in states that had included buprenorphine, naltrexone, or both for the treatment of opioid addiction in their Medicaid formulary in 2008. These data provide some evidence that the programs were correct in their awareness. Similar data on alcohol pharmacotherapies were not available. Future research should consider collecting policy data at the levels of programs and SSAs.
Finally, the focus of our study was adoption, defined as any prescription of these medications within treatment programs, rather than implementation, or the extent to which these medications were routinely used. Implementation was not measured, although recent data from a sample of privately funded programs indicated that the percentage of patients receiving medications within adopting organizations was limited (
21). Models of implementation are important directions for future research.
Conclusions
Our findings suggest that widespread adoption of medications in substance abuse treatment may be weakened by programs' lack of awareness about funding policies and a perceived lack of support by SSAs. Recent federal policy changes, such as the 2008 Wellstone-Domenici Parity Act and the 2010 Patient Protection and Affordable Care Act (health care reform), may have implications for the adoption of medications. How these policy changes will affect treatment programs that rely on governmental funding is not yet known, although there is some concern that the expansion of Medicaid eligibility under health care reform may cause states to cut behavioral health services to control overall spending (
30).
The evolving policy environment suggests that continued research is warranted. Nonetheless, these findings suggest that SSAs may play an important role in expanding the availability of medications for substance abuse treatment by conveying normative support for their use and designing and disseminating funding policies that help programs pay for their implementation.
Acknowledgments and disclosures
Support for this research was provided by grant 65111 from the Robert Wood Johnson Foundation's Substance Abuse Policy Research Program, grant R01DA014482 from the National Institute on Drug Abuse, and grant F32AA016872 from the National Institute on Alcohol Abuse and Alcoholism.
The authors report no competing interests.