Prevalence of autism spectrum disorder (ASD) has increased dramatically over the past ten years and is estimated to occur for every 1 in 68 children in the United States (
1). Coincident with this increase in prevalence is emerging evidence that ASD can be diagnosed reliably by age 18 months and that early intensive behavioral intervention can positively affect the core deficits of ASD (
1,
2). However, innovations in health care delivery systems that support early identification, definitive diagnosis, and treatment engagement for young children with ASD have not kept pace with advances in diagnosis and treatment (
2). A key weakness in this chain of services is access to specialty services required for definitive ASD diagnosis. Such a diagnosis is often a prerequisite for receipt of autism-specific services, and because access to specialty services is widely known to vary on the basis of socioeconomic status, race, and ethnicity, barriers to diagnostic services have the potential not only to affect a child’s long-term development but also to increase existing disparities in autism care (
3–
7).
One approach to reducing disparities related to timely diagnosis is patient navigation (
8), a time-limited model of lay case management that focuses on overcoming patient-specific barriers to care in the context of a complex health care system (
8). Patient navigation has a rich evidence base in cancer care (
8,
9); however, we know of no published report of its use with children with suspected ASD. We therefore conducted a pilot randomized controlled trial (RCT) to test the feasibility and acceptability of an adapted form of patient navigation, known as Family Navigation (FN). This intervention is targeted to low-income families from racial-ethnic minority groups with young children who are identified as at risk of having ASD. We assessed our ability to train individuals to deliver a standardized FN protocol and to recruit and retain families in the trial. We also assessed engagement of families with the FN intervention and the potential of FN to effect timely diagnosis of ASD. The original goal of this pilot RCT was not to detect statistically significant intervention effects. However, the serendipitous finding of the impact of FN on ASD diagnosis compelled us to expand our pilot approach to include group-to-group comparisons.
Methods
We enrolled 40 parent-children dyads who were referred by their primary care clinician for a diagnostic assessment for ASD due to concerns about social communication based on screening with the Modified Checklist for Autism in Toddlers or on clinical judgment. All children were referred to a single Developmental and Behavioral Pediatrics specialty clinic in an urban safety-net hospital. Usual care for an ASD diagnostic assessment involved three one-hour visits to conduct the assessment. All children had the first intake appointment scheduled and confirmed by a telephone call from the specialty clinic to the family. The clinic aimed to schedule the initial appointment for all children under three years of age within three weeks of referral. This policy was extended to all participants in this study, regardless of age. Children who did not attend their initial appointment did not have subsequent appointments scheduled unless the family contacted the clinic.
Study inclusion criteria were child age 15 months to six years and at least one characteristic empirically associated with risk of delay in ASD diagnosis (
3): income less than 200% of the federal poverty level, from a racial or ethnic minority group, foreign born, or maternal age <24 years. Children who had a previous diagnosis of ASD, resided more than 90 minutes from the clinic, or whose family spoke a language other than English, Spanish, or Haitian Creole were excluded. Study participants were recruited between December 2012 and March 2014. Eligible families were randomly allocated to FN or usual care according to a premade concealed schedule stored in sequentially numbered, opaque, sealed envelopes.
FN uses the core tenets of patient navigation (
6,
8,
10) but focuses more on the family than the patient and broadens the scope of navigation from medical system barriers to community and educational barriers. The goal of the navigator was to support the family to complete the child’s diagnostic assessment. Navigator tasks included exploring the family’s understanding of and need for the diagnostic assessment, identifying barriers to care and developing a plan to address them, completing documents required for the assessment, and ensuring that the family understands assessment results, recommendations, and available community resources (
11).
Navigators were three bilingual-bicultural women who had no prior experience with ASD. Their training included learning navigation competencies (
12), completing an online course created by the National Professional Development Center on Autism Spectrum Disorder (
13), and observing three ASD diagnostic assessments. On the basis of existing navigator research (
9), the model aimed for at least three in-person visits (in the home, the hospital outpatient clinic, or other place of the family’s choosing) and three telephone contacts between the navigator and the family but allowed for variation based on family needs. Navigators met weekly with investigators for supervision and case review.
The study’s primary outcome was time to diagnostic resolution (that is, being deemed to have ASD or not), as determined through medical record review. All children were followed for 365 days. Three secondary, family-focused outcomes—social support, perceived stress, and patient activation—were also assessed during a research interview conducted at baseline and two weeks after completion of the diagnostic assessment or, for families who did not complete the diagnostic process, 90 days after the child’s first scheduled clinic visit. [A more detailed description of the these outcomes, analytic methods, and findings is presented in an online supplement to this report.] Barriers to completing the diagnostic assessment were assessed during all FN contacts through open-ended questioning and were coded as individual, structural, or ASD specific. The Boston University Medical Center Institutional Review Board approved the study.
We compared baseline characteristics by intervention group. Using intention-to-treat analysis, we compared time to diagnosis between groups by creating a Kaplan-Meier plot and estimated the likelihood of completing the diagnostic assessment by using a Cox proportional hazards model. We verified the proportional hazards assumption by including a group × time interaction term in the Cox regression model.
Results
During the study period, 204 children under age six were referred for a diagnostic assessment for ASD; 140 families were screened for study eligibility; 64 were excluded before screening. Of the 140 families who were screened, 26 refused, 13 did not meet eligibility criteria, and 61 were potentially eligible but not enrolled because the time frame between referral and the scheduled first appointment at the specialty clinic was too short to obtain informed consent. The other 40 parent-child dyads consented to participate and were randomly assigned to study condition. One child, who already had an ASD diagnosis, was excluded postrandomization. [A CONSORT flowchart is available in the online supplement to this report.] Of the children randomly assigned, 40% (N=16) were Hispanic and 43% (N=17) were black, and most were male (78%, N=31); children’s mean±SD age was 3.11±.89 years. Forty-five percent (N=18) of the parents spoke a language other than English in the home. By intervention arm, there were no differences in demographic characteristics between participants.
In regard to navigation processes, the median number of in-person encounters with the navigator was four (range 1–9), and the median number of phone contacts was three (range 1–20). Seventeen of 20 families (85%) met the navigator for the target of three in-person visits, and visits lasted 70±22 minutes. Sixteen of the 20 intervention families (80%) reported five or more barriers to completing the diagnostic assessment. The most frequent barriers were understanding the objective of the diagnostic assessment, completing required paperwork, and scheduling and attending appointments.
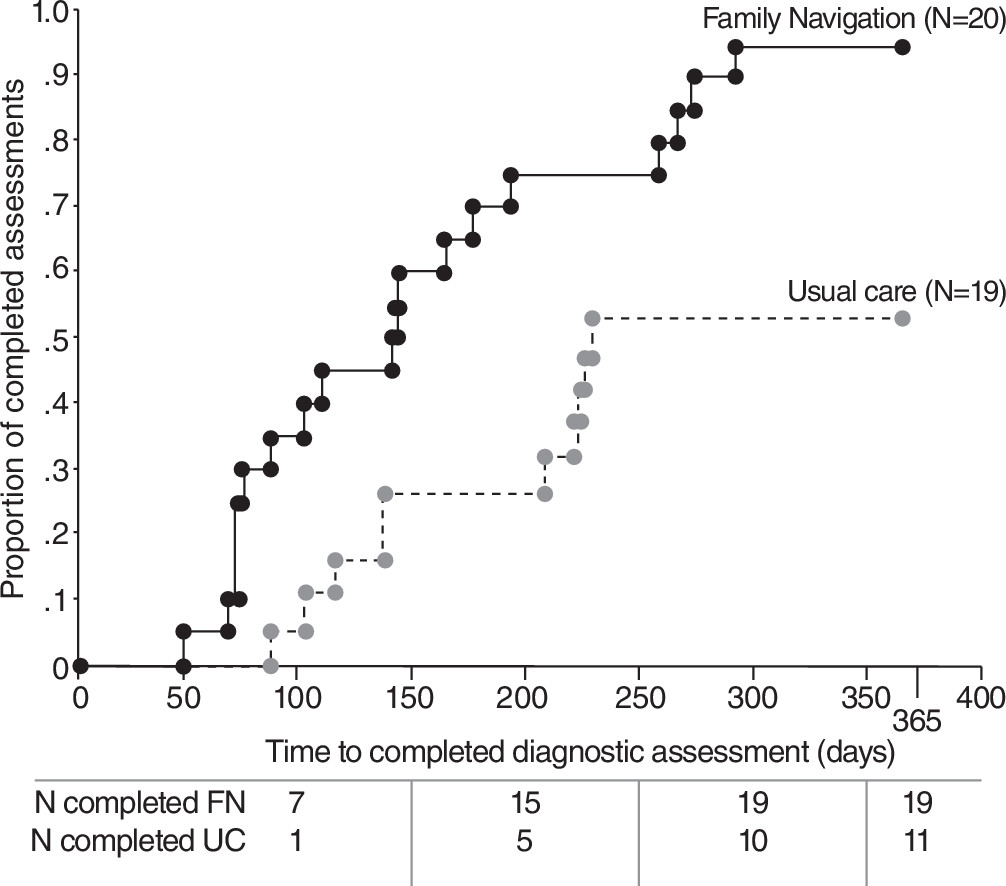
For the study’s primary outcome, at 365 days of follow-up, 19 of the 20 FN children (95%) had a completed diagnostic assessment, compared with 11 of 19 children who received usual care (58%). Forty-five percent (N=18) of FN children, compared with 16% (N=6) of usual care children, completed the three-visit assessment within four months. The FN families had greater than a 75% chance of completing the diagnostic assessment sooner than usual care families (hazard ratio=3.21, 95% confidence interval=1.47−6.98, p<.01) (
Figure 1). Among the 30 children who completed the diagnostic assessment, 12 (38%) received an ASD diagnosis (eight children in FN condition; four children in usual care); there were no differences between study arms in rate of ASD diagnosis. Of 18 children who did not receive an ASD diagnosis, the most common diagnoses were speech and language disorder (eight children) and global developmental delay (six children).
For secondary, family-focused outcomes—social support, perceived stress, and patient activation—standardized effect sizes (Cohen’s d) were moderate (.59), small (.20), and no effect (.02), respectively.
Discussion and Conclusions
The main finding of this pilot RCT, that children who received FN were significantly more likely than children who received routine clinic care to complete an ASD diagnostic assessment, was unanticipated. The effect of FN on time to ASD diagnosis identified in descriptive analysis convinced the study team to expand its analytic approach. Thus we conducted group-to-group comparisons that established statistical significance. Although further research is needed, particularly because of the limited sample size and single study site, the findings support that FN has the potential to address both structural and family barriers that impede timely ASD diagnosis. Because an ASD diagnosis conferred by a qualified clinician is often required for a child to receive intensive ASD services, completion of a diagnostic assessment becomes a significant predictor of timely access to recommended services and, ultimately, to improved developmental outcomes.
The feasibility and acceptability of FN deployed at the time of a referral for an ASD diagnostic evaluation were demonstrated in this study by the high level of family engagement with FN and study retention. Despite the stress and disruption of family routines associated with a possible ASD diagnosis, families engaged with their navigator and completed the targeted number of visits.
There were several limitations related to this study’s design, population, and implementation. In terms of the design and population, the study was conducted in a single clinic with specific procedures for scheduling and conducting ASD assessments. Further testing of FN in clinic settings that have different operating procedures is necessary to determine whether the impact of FN on diagnostic resolution can be replicated. The main limitation related to study implementation was the high proportion of children who were assessed for eligibility (61 of 140, 44%) but were not enrolled in the study because the period between referral and the first scheduled appointment of the diagnostic assessment was too short to allow time for consent. To assess potential selection bias, we conducted a chart review of these 61 children that suggested that, on discernible demographic characteristics, they were similar to our randomized sample and the proportion who completed the diagnostic assessment (45%) was similar to that for children who were randomly assigned to usual care. The study also did not collect detailed data on how FN worked with families to address barriers to diagnosis, which would allow us to further understand the intervention mechanism.
Despite these limitations, the evidence of FN’s potential to improve timely diagnosis of young children at risk of ASD supports further evaluation of this intervention. With growing evidence of the efficacy of early intensive behavioral interventions for ASD, FN, if replicable, may represent a promising public health strategy to reduce disparities in timely access to ASD services.
Acknowledgments
The authors acknowledge the families who participated in the study and the staff of the specialty clinic where the autism diagnostic assessments were conducted.